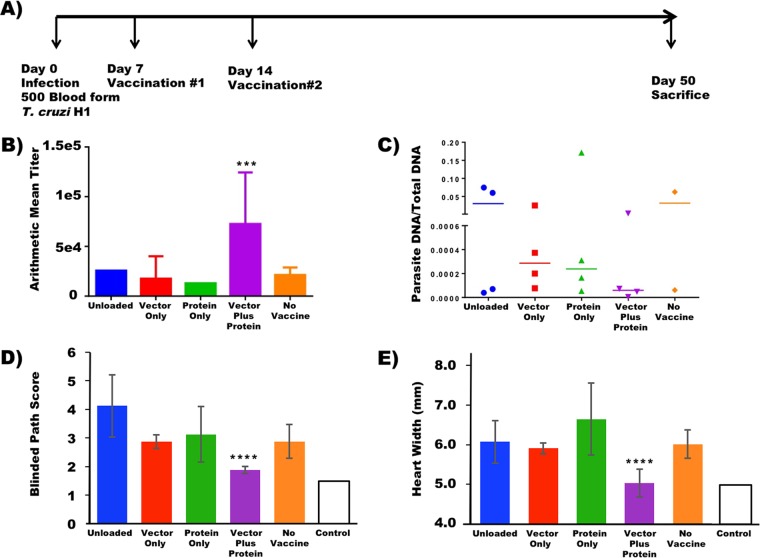
FIG 4.
DC vaccines transduced with the dnSHP adenoviral construct and loaded with the recombinant Tc24 protein reduce the T. cruzi parasite burden. (A) To test the therapeutic efficacy of the vaccine, naive BALB/c mice were infected intraperitoneally with 500 blood-form trypomastigotes. At 7 dpi, the mice were therapeutically immunized intraperitoneally with 250,000 DCs transduced with the dnSHP vector alone, DCs loaded with the recombinant Tc24 protein only, and DCs transduced with the dnSHP vector and simultaneously loaded with the recombinant Tc24 protein. Unloaded DCs served as controls. Boost vaccination was given at 14 dpi. At 50 dpi, all remaining mice were sacrificed and analyzed. (B) Serum antibody titers analyzed for the mice at the end of the study demonstrate that mice treated with vector-plus-protein-loaded DCs exhibited significantly elevated Tc24-specific IgG1 antibody titers (P < 0.004). (C) Endpoint cardiac parasite DNA was quantified by qPCR analysis using T. cruzi-specific primers. A trend toward low/no detectable parasite DNA (P = 0.08) was observed among mice that received vector-plus-protein-loaded DCs compared to the other groups. (D and E) H&E staining was performed on cardiac tissue at the end of the study, and pathological scores on a scale of 0 to 5 were given for lymphocytic infiltration, with 5 being maximum infiltration. Randomized and blind pathological scoring demonstrated that mice which received vector-plus-protein-loaded DCs exhibited substantially lower pathological index scores, some on par with those of uninfected mice (P < 0.0006). The endpoint cardiac size (enlargement) as assessed by cross-sectional distance was nearly 20% smaller (5.04 mm versus 6.16 mm; P < 0.0001) among mice that received vector-plus-protein-loaded DCs and similar to that of uninfected mice. For all panels, error bars indicate SEM.