Abstract
In this time of massive global change, species are now frequently interacting with novel players. Greater insight into the impact of these novel interactions on traits linked to fitness is essential, because effects on these traits can hinder population existence or promote rapid adaptation. Sexually selected weapons and ornaments frequently influence fitness and often have heightened condition-dependence in response to nutrition. Condition-dependence in response to different ecological conditions, a form of developmental plasticity, may be responsible for much of the intraspecific variation in sexually selected ornaments and weapons in wild populations. Here we examined the consequences of developing on a novel plant for the expression of size and shape in the leaf-footed cactus bug Narnia femorata (Hemiptera: Coreidae). The males of this species possess enlarged, sexually dimorphic femurs on their hind legs. These legs are used as weapons in male–male contests. Females are typically larger in overall body size. Our study revealed that developing upon a novel host can lead to pronounced phenotypically plastic change in sexually dimorphic traits. Male hind femurs were greatly impacted by the novel diet to the extent that the sexual dimorphism in hind femurs was lost. Further, dimorphism in body size increased, as males became tiny adults while females better maintained their body size. These patterns underscore the complex effects that novel species interactions may have on sexual phenotypes.
Keywords: allometry, body shape, condition-dependence, phenotypic plasticity, scaling
1. Introduction
The giant antlers of elk, the major chela of the fiddler crab and the horns of the rhinoceros beetle are some of the most iconic traits in the animal kingdom and the result of sexual selection [1,2]. While these weapons are highly variable across species, they also show surprising variation within species [3,4]. The within-species variation often reflects their heightened sensitivity to condition [5,6]. In many cases, the variation in weapon and ornament size may serve as an honest indicator of differences between individuals in quality [7,8], and therefore it may be of ample importance in male–male contests and mate choice.
The heightened condition-dependence in ornaments and weapons is likely to be an evolutionary consequence of a history of directional sexual selection [6–8]. Heightened condition-dependence can be thought of as a type of developmental plasticity, in which trait expression becomes disproportionately linked to the amount of nutritional/metabolic resources available to an individual, when compared with other traits [5,7,9]. In other words, all morphological traits exhibit some level of condition-dependence, but exaggerated sexual traits are expected to be more sensitive due to their disproportionate size and cost. Experimental studies of condition-dependence have illustrated that sexually selected traits are often greatly reduced in individuals experiencing poor diets or other stressors [5,6,8,10–14], and suggest intriguing consequences for wild populations in this time of massive environmental change. Introduced and invasive plants are now a common sight globally, and in many cases, herbivorous insects feed upon these new resources. Yet virtually no research has explicitly addressed the impact of shifts in diet on the expression of sexually selected traits [15], especially relative to other traits and homologous traits in the opposite sex [16]. The impacts of these host shifts on morphology may reduce individual fitness and alter population persistence [17,18].
Here, we simulated a natural host plant shift to examine the response in a sexually selected weapon, the homologous traits in females and other morphological characteristics for comparison using the leaf-footed cactus bug, Narnia femorata. Males of this species are slightly smaller than females in body size [14,19], but possess enlarged hind legs (sexually selected traits) used in male–male contests [20,21]. Previous work has demonstrated that seasonal changes in nutrition have striking effects on weapon size, body shape [19], internal anatomy [22] and sexual dimorphism in this species [14]. These results suggest that these traits may be highly susceptible to dietary stress that comes from a novel host. Based on these patterns, we predicted that male insects would not only develop as small adults on the novel host, but they would also bear disproportionately smaller sexually selected traits when compared with female homologous traits and non-sexual traits. We further predicted that novel host use would affect the overall body shape of both sexes, as has been previously found in other insects raised on artificial diets [6,23]. We present evidence that a shift onto a novel host plant can lead to a reduction in the size of a sexually selected weapon, so that the sexual dimorphism in this trait is lost.
2. Material and methods
(a). Study organism and hosts
The cactus-feeding leaf-footed bug N. femorata Stål (Hemiptera: Coreidae) is native to the US southwest through Mexico; it has known association with at least four genera and 13 species of cacti [24]. It has established a population in central Florida, where it primarily feeds on the native prickly pear Opuntia mesacantha [25]. Females lay clutches of eggs on cactus spines, and offspring can develop to sexual maturity on one plant. Males establish territories on cacti and defend their territories against other conspecifics using their hind legs (weapons). Larger males are more likely to be dominant over smaller ones when defending their territories [20,21]. Developmental environment is important for the expression of body size [19], weapon size [14,26] and the degree of sexual dimorphism in these traits [14]. Males that develop without ripe cactus fruit become smaller adults with disproportionately reduced hind femurs [14,26].
We used the Mexican endemic O. robusta to test the effects of a novel host on N. femorata sexually selected traits. This cactus species is widely cultivated in North America and invasive in Australia, yet N. femorata has no known history of feeding on this plant [27]. The novel host fruit appears to impose a bigger physical barrier for the cactus bugs as its pulp and seeds are deeper into the fruit (electronic supplementary material, figure S1). To examine these structural differences between the fruits, we measured the thickness of the walls by cutting the fruits longitudinally and recording the distance between the outside surface and the pulp (electronic supplementary material, figure S1). We measured 20 local fruits and 10 novel fruits haphazardly chosen from our experiment stock.
(b). Rearing and experimental design
A colony of Florida N. femorata was established in a greenhouse using adults collected in September 2013 from the Ordway-Swisher Biological Station (29.4° N, 82.0° W) in north-central Florida. Insects were haphazardly paired and kept in plastic deli containers covered by a plastic lid with a 20 cm2 mesh window, with 4 cm of topsoil, and a cactus pad (O. mesacantha) with ripe fruit attached. Females laid eggs for three weeks or until they reached 40 eggs. First-generation nymphs from 10 families were split equally across the two different host plant fruit treatments (O. mesacantha and O. robusta). Nymphs were raised in groups of 5–8 on a single fruit and cactus pad, because they commonly aggregate as juveniles.
(c). Bug measurements
We froze mature adults, and then we separated hind and front legs from the body to facilitate the measuring procedure. We used a digital camera (Canon EOS 50D) attached to a dissecting microscope (Leica M165 C) to photograph all the extremities and body. The software ImageJ [28] was used for the linear measurements of the following (measurements of left and right were averaged where applicable): beak length, head length, pronotum width (PW), front femur length (FFL), hind femur length and hind femur width. The term ‘beak’ can be employed for the straw-like mouthparts of hemipterans [29]. We measured the area of the hind tibia and femur. These traits were chosen to obtain data on metric (body size), feeding (mouthparts) and sexually selected traits. We used PW as a proxy for body size because in this species it is highly correlated with overall body size (electronic supplementary material, table S1) [19,20]. Hind femur width (r = 0.94) and hind femur length (r = 0.946) are used in figure 1e to illustrate the high degree of correlation with hind femur area (HFA), and to further demonstrate the sex-specific pattern of condition-dependence. Neither measurement was used in statistical analysis because HFA includes these measurements.
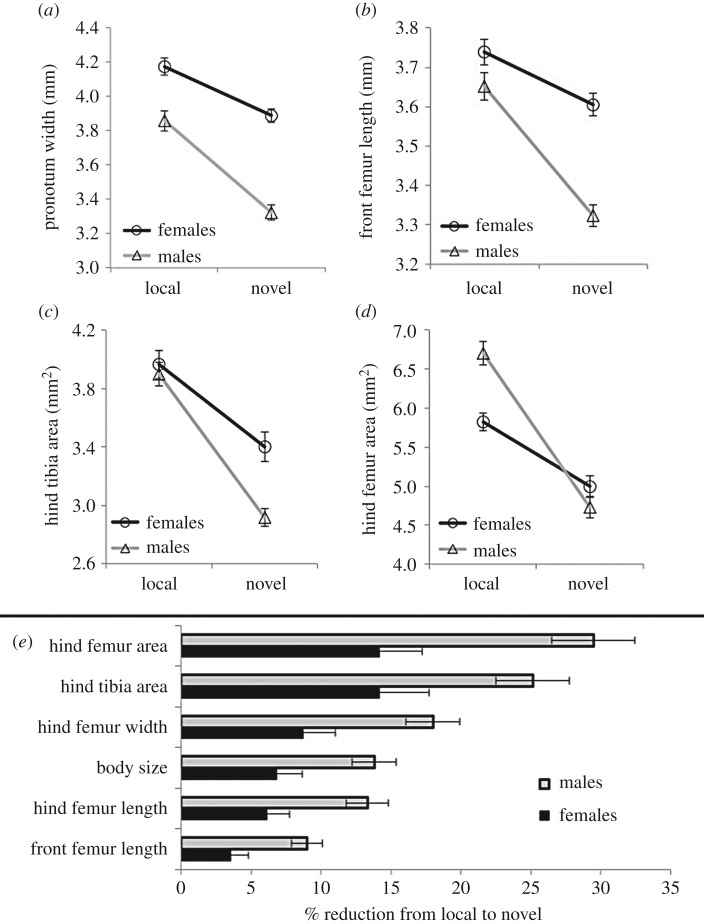
Figure 1.
Sexual dimorphism changes significantly across hosts. (a) Mean (±s.e.) body size (pronotum width), (b) front and (c,d) hind leg traits sizes, for both sexes across hosts. (e) Reduction in absolute mean size (±s.e.) across hosts for the traits measured, for females (black bars) and males (grey bars).
(d). Statistical analysis
All analyses were conducted with IBM SPSS v. 22. We used a factorial analysis of variance (ANOVA) to test for host plant and sex effects and their interactions on all the morphological traits. For statistical analyses we considered each individual insect independent, as the within-cup (including same fruit) variation in individual size is large [30] and therefore of biological importance. We also conducted separate analyses using cup (family group) means to account for the possible non-independence of insects from the same cup and obtained qualitatively similar results.
We tested for host effects on scaling relationships using analyses of covariance (ANCOVA) independently for each of the traits FFL, hind tibia area and HFA, with PW as the covariate, separately for each sex. This test allows detecting differences between treatments in slopes (b) and elevations (intercept) of the linear regressions accounting for body size (PW). We used log–log ordinary least squares (OLS) regression method to compare slopes (b) as this method facilitates statistical testing of the relationship between X and Y [31] (see also further arguments for the use of this method [32–36]).
To further evaluate changes in shape due to the novel host, we used principal component analysis (PCA). Measurements of beak and head length were used in building the PCA to provide more precision in estimating size and shape of individuals; these measurements were not analysed elsewhere. The six measured traits are correlated (see electronic supplementary material, table S1); therefore, it is useful to extract new uncorrelated variables. We ran a single PCA so that we could make comparisons across the sexes and treatments; we used the correlations matrix and the varimax rotation to obtain our factors. We reduced our variable number (n = 6) down to two. Principal component (PC) 1 and PC 2 were extracted to account for 88% of the variation in the data. We then used these components to map in two dimensions the change in overall shape caused by the novel host. The first PC factor explained 76% of the variation in the data and was mainly driven by hind leg traits (electronic supplementary material, table S2). The second PC factor explained 12% and it was driven by beak length and body size (head length and PW). To test for sex and host effects on PC factors, we used two-way ANOVAs.
3. Results
The novel fruit, O. robusta, has thicker walls (mean = 6.30 mm, s.e. = 0.48; electronic supplementary material, figure S1), meaning that the pulp is deeper than in the local O. mesacantha fruits (mean = 3.94 mm, s.e. = 0.35; t-test: t = 3.9, d.f. = 26, p = 0.001). As predicted, N. femorata raised on the novel host, O. robusta, had lower survivorship than those on the local host, O. mesacantha (46% survivorship on O. robusta versus 71% survivorship on O. mesacantha, respectively; χ² = 18.7, d.f. = 1, p < 0.0001). Survivors on the novel host had slower growth, 58% longer development time from second instar to adulthood for both sexes (F1,164 = 128.6, p < 0.0001), with no significant differences between males and females (F1,164 = 3.16, p = 0.077) on either host (electronic supplementary material, figure S2).
We found drastic and somewhat unexpected changes in the morphology of N. femorata when we simulated a natural host plant shift. Most surprisingly, absolute (mean size) male-biased sexual dimorphism of hind femurs was lost on the novel diet (table 1, figure 1d). In other words, on the natural diet, males typically have larger hind legs than females, but on the novel diet weapon dimorphism disappeared. In general, the negative effect of the novel host on male trait size was greater than the effect on female size, both in absolute trait values and also relative to overall body size (figure 1). Male mean overall size (PW) was reduced by 13.7% on the novel host, while female size only declined by 6.8% (figure 1a,e). Furthermore, cactus bugs reared on the novel host developed smaller front and hind legs than those reared on the local host. But, as predicted, the magnitude of change across hosts widely varied between traits (table 1, figure 1). The focal traits responded to the novel host differently, from relatively insensitive (FFL) to highly sensitive (HFA) in this new environment (figure 1e). This effect was more pronounced in males than females, as indicated by significant sex × host interactions on the morphological traits (table 1).
Table 1.
Separate analyses of variance (ANOVA) by morphological trait, for effects of sex, host and sex × host interaction. F-values are shown with corresponding p-values. PW, pronotum width; FFL, front femur length; HTA, hind tibia area; HFA, hind femur area. Note: d.f.= 1, 163.
| sex |
host |
sex × host |
||||
|---|---|---|---|---|---|---|
| trait | F-value | p-value | F-value | p-value | F-value | p-value |
| PW | 78.8 | <0.0001 | 67.6 | <0.0001 | 6.25 | 0.013 |
| FFL | 30.7 | <0.0001 | 48.2 | <0.0001 | 8.7 | 0.004 |
| HTA | 11.4 | 0.001 | 77.0 | <0.0001 | 7.3 | 0.007 |
| HFA | 4.15 | 0.043 | 88.7 | <0.0001 | 15.0 | <0.0001 |
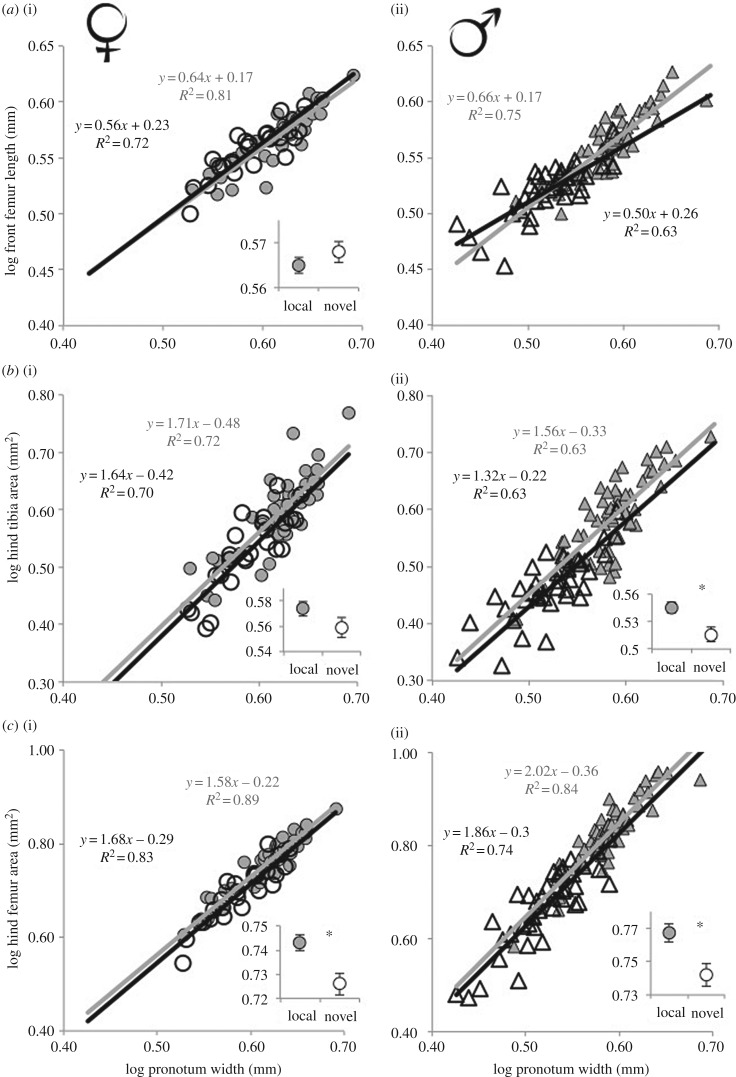
The changes in body shape across diets were due to changes in the size of the sexually selected traits relative to body size (tables 2 and 3, figure 2). The host plant species fed upon during juvenile development affected the allometric slope (host × PW interaction) of male FFL (table 2, figure 2a(ii)). Owing to this difference in slope for male FFL an analysis of changes in intercept was not appropriate for this trait (table 3, figure 2). Differences in allometric intercepts vary according to the placement of the intercept when trait slopes vary. Female FFL, relative to body size, was not affected by host species (table 3). The novel host plant negatively affected the relative size of male HTA (figure 2b(ii)) and of HFA for both sexes (figure 2c).
Table 2.
Changes in slope. The trait size to body size relationship (slope) was affected by host (H) only for male front femur length (FFL). Separate ANCOVA results for the effect of host on allometric slope for front femur length, hind tibia area (HTA) and hind femur area (HFA) with pronotum width (PW) as covariate for Narnia femorata adults. F-values are shown with corresponding p-values; probabilities less than 0.05 are highlighted in italics. [d.f.].
| females |
[1, 64] |
males |
[1, 95] |
||
|---|---|---|---|---|---|
| sex trait | F-value | p-value | F-value | p-value | |
| FFL | PW | 195.9 | <0.0001 | 218.2 | <0.0001 |
| host | 1.16 | 0.29 | 3.93 | 0.05 | |
| H × PW | 1.03 | 0.31 | 4.25 | 0.042 | |
| HTA | PW | 138.4 | < 0.0001 | 155.9 | <0.0001 |
| host | 0.13 | 0.72 | 0.65 | 0.42 | |
| H × PW | 0.07 | 0.79 | 1.1 | 0.3 | |
| HFA | PW | 392.7 | <0.0001 | 364.5 | <0.0001 |
| host | 0.62 | 0.43 | 0.31 | 0.58 | |
| H × PW | 0.37 | 0.54 | 0.61 | 0.44 |
Table 3.
Changes in intercept. The novel host affected negatively the size of hind femur area (HFA) for both sexes, and for male hind tibia area (HTA), relative to body size (intercept). Separate ANCOVA results for the effect of host on the intercept for front femur length (FFL), hind tibia area and hind femur area with pronotum width (PW) as covariate for Narnia femorata adults, separating by sex. F-values are shown with corresponding p-values; probabilities less than 0.05 are highlighted in italics. Male FFL was not evaluated as the novel host caused a difference in allometric slope (table 2). [d.f.].
| females |
[1, 65] |
males |
[1, 96] |
||
|---|---|---|---|---|---|
| sex factor | F-value | p-value | F-value | p-value | |
| FFL | PW | 230.7 | <0.0001 | — | — |
| host | 1.1 | 0.31 | — | — | |
| HTA | PW | 155.4 | <0.0001 | 158.9 | <0.0001 |
| host | 2.2 | 0.14 | 7.16 | 0.009 | |
| HFA | PW | 436.4 | <0.0001 | 370.7 | <0.0001 |
| host | 8.5 | 0.005 | 6.4 | 0.013 |
Figure 2.
The novel host negatively affected the scaling relationships of the three focal traits, except for female front femur length and hind tibia area. The slope of male front femur length (FFL) was significantly affected by the novel host. Trait size relative to body size (intercept) was affected by the novel host for male hind tibia area and hind femur area in both sexes. Allometry for (a) front femur length and hind leg traits, (b) hind tibia area and (c) hind femur area, for females (triangles) and males (circles) of Narnia femorata on the local host (grey regression line = Opuntia mesacantha) and novel host (black/hollow regression line = O. robusta). Insets show the least-square estimates (±s.e.) for each trait at the mean body size for each sex. Asterisks indicate significant differences (p < 0.05) in allometric intercepts between hosts. Notes: owing to the difference in slope between hosts for male FFL we did not test for differences in intercept for that trait in males; females on the left panels, males on the right panels.
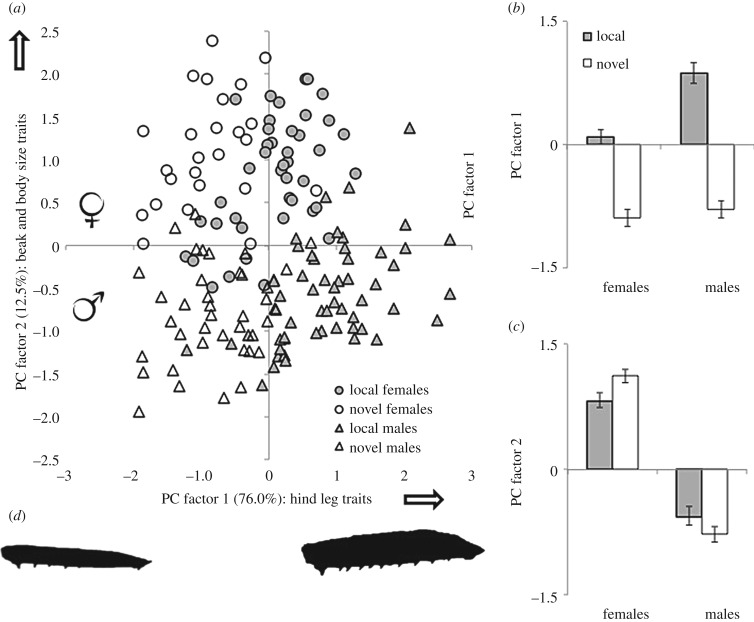
The PCA (electronic supplementary material, tables S1 and S2; figure 3), performed to evaluate the whole-body consequences of the novel host beyond the individual trait effects, supported the previously described results that the novel host diet during nymphal development had a substantial effect on the overall shape of the cactus bugs. Additionally, the PCA highlights the higher condition-dependence of male body shape. This analysis confirmed that the effect was mostly due to decreased hind leg dimorphism (male weapons; PC factor 1; figure 3a,b), but also increased sexual size dimorphism (PC factor 2; figures 1 and 3a,c).
Figure 3.
Changes in body shape due to a novel host, for both sexes of Narnia femorata. (a,b) PC (principal component) factor 1: the novel host causes a reduction of hind leg traits (tibia and femur), the effect is more pronounced for males (two-way ANOVAdf = 1,158: sex F = 15.4, p < 0.0001; host F = 138.5, p < 0.0001; interaction F = 9.2, p = 0.003). (a,c) PC factor 2: the novel host increases the differences in body size traits between the sexes (two-way ANOVAdf = 1,158: sex F = 100.5, p < 0.0001; host F = 0.23, p = 0.63; interaction F = 6.23, p = 0.014). The white arrows indicate the direction towards which traits are bigger. (d) Male hind femur outlines are shown to scale, illustrating the difference between smallest and largest.
4. Discussion
An increasing number of studies suggest that sexually selected traits are often highly condition-dependent [5,6,8,10,11]. Furthermore, much work has shown the generally detrimental effects of novel hosts on insects [37,38]. This study connects those fields of research. Overall, we found that the novel host plant reduced survivorship. Those adults that survived were smaller on the novel host (figure 1a). The effects of the novel host differed in magnitude between male sexual and non-sexual traits, and between homologous traits in males and females. These sex-specific responses resulted in changes in sexual dimorphism. The absolute sexual dimorphism in HFA, a weapon used in male–male contests, was lost (figures 1d and 3b). These results highlight the importance of an adequate natural nutritional environment for the full expression of sexually selected traits, and suggest negative implications of novel environments for success in male–male contests.
Using only its natural diet in central Florida, previous research with N. femorata has found a fluctuation in sexual dimorphism of male weapons seasonally with changes in host plant phenology [14,26]. This earlier work illustrated how both sexes were heavily affected by diet quality, and that the male hind femur traits were affected disproportionately more than other traits. Still, the size of male hind femurs on the local diet was larger than the female homologue even when diet quality declined [14]. Here, we found that the novel host increased sexual dimorphism in body size, at a level not previously seen [14], while simultaneously erasing the difference between the sexes in the expression of HFA (figure 1d). Plasticity due to the novel host effectively created new sexual phenotypes (figure 3). In addition to the significant changes in absolute trait sizes, the novel host also caused negative changes in relative size (figure 2). Both male hind leg traits and female hind femurs expressed smaller sizes relative to body size when the insects developed on the novel host. Even though the changes in scaling relations are relatively small they exacerbate the negative effects and add complexity to the new interaction between herbivore and host.
The changes in morphology seen here suggest that novel hosts may have complex effects on selection and the evolutionary response to selection. For example, signalling of male genetic quality may be disrupted when populations are in the process of shifting onto a new host. Furthermore, shifts in trait size distributions, such as can occur when a novel host plant is used, may shift the strength or direction of selection [39]. Such a change can occur because the relationship between the size of sexually selected traits and fitness is sometimes complex, with multiple peaks and valleys [40,41]. The new maximum trait value after a host shift could, for example, end up in a fitness valley. Next, the agents of sexual selection, mate choice and male–male competition may themselves change as environments transform [42,43]. Differences in how small males fight, for example, could lead to selection on different morphological elements of the weapon, and thus could lead to evolutionary changes in weapon shape. Finally, the environment, sexual selection and genetic variance may have positive or negative covariances, which can accelerate or hamper trait evolution [44–46]. In some cases, new environments may reveal cryptic genetic variance in sexually selected traits, enabling rapid responses to selection [47]. For example, Husby et al. [46] found that the strength of selection and expression of genetic variance in great tits (Parus major) are positively linked with increased spring temperature, potentially leading to an acceleration of evolution.
The major goal of this study was to experimentally examine the phenotypic consequences of developing on a novel host. Previous work in this species has indicated the heightened sensitivity of sexually selected traits and sexual dimorphism to differences in nutrition [14,19,26]. Thus, the patterns of phenotypic expression documented here are probably a result of phenotypic plasticity in traits due to good or poor nutrition. However, it is worth considering that a component of the differences across treatments in morphological traits may be due to selection via differential survival in this single generation. Survivorship on the novel host (O. robusta) was 46% versus 71% on the local host (O. mesacantha). On the novel host, the largest of existing nymphs may have died, for example, leaving only the small individuals. However, from visual inspection of figures 2 and 3, it is clear that males that developed in the novel host occupy a different phenotypic space than males that fed from the local host—at the lower and higher ends of all trait size distributions. Such profound differences are probably due substantially to the effects of nutrition.
Phenotypic plasticity has a major role in sexual selection dynamics, as the expression of sexually selected traits is intimately tied with the environmental conditions surrounding the development of the bearers [48]. In this study, we found evidence of how plasticity on a novel host can cause drastic changes in overall sexual dimorphism, including the complete loss of dimorphism in a sexually selected weapon. The link between sexual dimorphism and condition-dependence has been previously explored in two holometabolous species: the flies Prochyliza xanthostoma (Piophilidae) [5] and Telostylinus angusticollis (Neriidae) [6]. Similar to our findings, these studies illustrate the strong link between the condition-dependence of sexually selected traits and the plasticity of sexual dimorphism, though these studies used only artificial diets [5,6]. In both those experiments, as in the present one, the data suggest that the level of condition-dependence was highest for male sexually selected traits, and also that body shape was more condition-dependent in males than females [5,6]. Thus, our results expand the understanding of the link between condition-dependence and sexual dimorphism using a hemimetabolous insect, and create a bridge onto the field of new insect–plant interactions and the consequences of biological invasions. The effects of the novel host are by far much more pronounced than those that occur seasonally with variation in their natural diet [14,19,26]. Our study suggests that novel hosts could alter the sexual selection dynamics of herbivorous insects.
Supplementary Material
Supplementary Material
Acknowledgements
Savannah Nease provided invaluable help during the rearing period. Marc Branham, Emilio Bruna, Johel Chaves-Campos, Daniel Sasson, Colette St Mary and two anonymous reviewers provided comments and feedback on earlier drafts.
Data accessibility
The dataset supporting this article have been uploaded to Dryad Digital Repository (doi:10.5061/dryad.5p19k) [49].
Authors' contributions
P.E.A. and C.W.M. conceived the study and conducted fieldwork. P.E.A. conducted the experiment. Both authors analysed the data, participated in manuscript preparation and have given final approval for publication.
Competing interests
We have no competing interests.
Funding
This work was supported by grants from the National Science Foundation, IOS- 092685 and IOS-1553100 to C.W.M.
References
- 1.Darwin C. 1871. The descent of man and selection in relation to sex. London, UK: Murray. [Google Scholar]
- 2.Andersson MB. 1994. Sexual selection. Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Emlen DJ, Nijhout HF. 2000. The development and evolution of exaggerated morphologies in insects. Annu. Rev. Entomol. 45, 661–708. ( 10.1146/annurev.ento.45.1.661) [DOI] [PubMed] [Google Scholar]
- 4.Emlen DJ. 2008. The evolution of animal weapons. Annu. Rev. Ecol. Evol. Syst. 39, 387–413. (doi:30245169) [Google Scholar]
- 5.Bonduriansky R, Rowe L. 2005. Sexual selection, genetic architecture, and the condition dependence of body shape in the sexually dimorphic fly Prochyliza xanthostoma (Piophilidae). Evolution 59, 138–151. ( 10.1111/j.0014-3820.2005.tb00901.x) [DOI] [PubMed] [Google Scholar]
- 6.Bonduriansky R. 2007. The evolution of condition-dependent sexual dimorphism. Am. Nat. 169, 9–19. ( 10.1086/510214) [DOI] [PubMed] [Google Scholar]
- 7.Rowe L, Houle D. 1996. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. Lond. B 263, 1415–1421. ( 10.1098/rspb.1996.0207) [DOI] [Google Scholar]
- 8.Emlen DJ, Warren IA, Johns A, Dworkin I, Lavine LC. 2012. A mechanism of extreme growth and reliable signaling in sexually selected ornaments and weapons. Science 337, 860–864. ( 10.1126/science.1224286) [DOI] [PubMed] [Google Scholar]
- 9.Nur N, Hasson O. 1984. Phenotypic plasticity and the handicap principle. J. Theor. Biol. 110, 275–297. ( 10.1016/S0022-5193(84)80059-4) [DOI] [Google Scholar]
- 10.David P, Bjorksten T, Fowler K, Pomiankowski A. 2000. Condition-dependent signalling of genetic variation in stalk-eyed flies. Nature 406, 186–188. ( 10.1038/35018079) [DOI] [PubMed] [Google Scholar]
- 11.Cotton S, Fowler K, Pomiankowski A. 2004. Condition dependence of sexual ornament size and variation in the stalk-eyed fly Cyrtodiopsis dalmanni (Diptera: Diopsidae). Evolution 58, 1038–1046. ( 10.1111/j.0014-3820.2004.tb00437.x) [DOI] [PubMed] [Google Scholar]
- 12.Delcourt M, Rundle HD. 2011. Condition dependence of a multicomponent sexual display trait in Drosophila serrata. Am. Nat. 177, 812–823. ( 10.1086/659949) [DOI] [PubMed] [Google Scholar]
- 13.Gosden TP, Chenoweth SF. 2011. On the evolution of heightened condition dependence of male sexual displays. J. Evol. Biol. 24, 685–692. ( 10.1111/j.1420-9101.2010.02205.x) [DOI] [PubMed] [Google Scholar]
- 14.Miller CW, McDonald GC, Moore AJ. 2016. The tale of the shrinking weapon: seasonal changes in nutrition affect weapon size and sexual dimorphism, but not contemporary evolution. J. Evol. Biol. 29, 2266–2275. ( 10.1111/jeb.12954) [DOI] [PubMed] [Google Scholar]
- 15.Svensson EI, Gosden TP. 2007. Contemporary evolution of secondary sexual traits in the wild. Funct. Ecol. 21, 422–433. ( 10.1111/j.1365-2435.2007.01265.x) [DOI] [Google Scholar]
- 16.Cotton S, Fowler K, Pomiankowski A. 2004. Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proc. R. Soc. Lond. B 271, 771–783. ( 10.1098/rspb.2004.2688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strauss SY, Lau JA, Carroll SP. 2006. Evolutionary responses of natives to introduced species: what do introductions tell us about natural communities? Ecol. Lett. 9, 357–374. ( 10.1111/j.1461-0248.2005.00874.x) [DOI] [PubMed] [Google Scholar]
- 18.Forister ML, Scholl CF. 2012. Use of an exotic host plant affects mate choice in an insect herbivore. Am. Nat. 179, 805–810. ( 10.1086/665647) [DOI] [PubMed] [Google Scholar]
- 19.Gillespie SR, Tudor MS, Moore AJ, Miller CW. 2014. Sexual selection is influenced by both developmental and adult environments. Evolution 68, 3421–3432. ( 10.1111/evo.12526) [DOI] [PubMed] [Google Scholar]
- 20.Procter DS, Moore AJ, Miller CW. 2012. The form of sexual selection arising from male–male competition depends on the presence of females in the social environment. J. Evol. Biol. 25, 803–812. ( 10.1111/j.1420-9101.2012.02485.x) [DOI] [PubMed] [Google Scholar]
- 21.Nolen ZJ, Allen PE, Miller CW. 2017. Seasonal resource value and male size influence male aggressive interactions in the leaf foot cactus bug, Narnia femorata. Behav. Process 138, 1–6. ( 10.1016/j.beproc.2017.01.020) [DOI] [PubMed] [Google Scholar]
- 22.Joseph PN, Sasson DA, Allen PE, Somjee U, Miller CW. 2016. Adult nutrition, but not inbreeding, affects male primary sexual traits in the leaf-footed cactus bug Narnia femorata (Hemiptera: Coreidae). Ecol. Evol. 6, 4792–4799. ( 10.1002/ece3.2246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassidy EJ, Bath E, Chenoweth SF, Bonduriansky R. 2013. Sex-specific patterns of morphological diversification: evolution of reaction norms and static allometries in neriid flies. Evolution 68, 368–383. ( 10.1111/evo.12276) [DOI] [PubMed] [Google Scholar]
- 24.Vessels HK, Bundy CS, McPherson JE. 2013. Life history and laboratory rearing of Narnia femorata (Hemiptera: Heteroptera: Coreidae) with descriptions of immature stages. Ann. Entomol. Soc. Am. 106, 575–585. ( 10.1603/AN13084) [DOI] [Google Scholar]
- 25.Baranowski RM, Slater JA. 1986. Coreidae of Florida (Hemiptera, Heteroptera) Gainesville, FL: Florida Department of Agriculture and Consumer Services, Division of Plant Industry, Bureau of Entomology.
- 26.Sasson DA, Munoz PR, Gezan SA, Miller CW. 2016. Resource quality affects weapon and testis size and the ability of these traits to respond to selection in the leaf-footed cactus bug, Narnia femorata. Ecol. Evol. 6, 2098–2108. ( 10.1002/ece3.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janczur MK, et al. 2014. Chemical and physical defense traits in two sexual forms of Opuntia robusta in central eastern Mexico. PLoS ONE 9, e89535 ( 10.1371/journal.pone.0089535) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasband WS. 2011. Imagej. Bethesda, MD: US National Institutes of Health: See http://rsb.info.nih.gov/ij. [Google Scholar]
- 29.Carroll SP, Boyd C. 1992. Host race radiation in the soapberry bug: natural history with the history. Evolution 46, 1052–1069. ( 10.1111/j.1558-5646.1992.tb00619.x) [DOI] [PubMed] [Google Scholar]
- 30.Allen PE, Miller CW. In preparation. Sex-specific consequences of juvenile social environment on development rate and body size.
- 31.Warton DI, Wright IJ, Falster DS, Westoby M. 2006. Bivariate line-fitting methods for allometry. Biol. Rev. 81, 259–291. ( 10.1017/S1464793106007007) [DOI] [PubMed] [Google Scholar]
- 32.Smith RJ. 2009. Use and misuse of the reduced major axis for line-fitting. Am. J. Phys. Anthropol. 140, 476–486. ( 10.1002/ajpa.21090) [DOI] [PubMed] [Google Scholar]
- 33.Eberhard WG. 2009. Static allometry and animal genitalia. Evolution 63, 48–66. ( 10.1111/j.1558-5646.2008.00528.x) [DOI] [PubMed] [Google Scholar]
- 34.Pélabon C, Firmat C, Bolstad GH, Voje KL, Houle D, Cassara J, Le Rouzic A, Hansen TF. 2014. Evolution of morphological allometry. Ann. NY Acad. Sci. 1320, 58–75. ( 10.1111/nyas.12470) [DOI] [PubMed] [Google Scholar]
- 35.Voje KL, Hansen TF, Egset CK, Bolstad GH, Pélabon C. 2014. Allometric constraints and the evolution of allometry. Evolution 68, 866–885. ( 10.1111/evo.12312) [DOI] [PubMed] [Google Scholar]
- 36.Kilmer JT, Rodriguez RL. 2016. Ordinary least squares (OLS) regression is indicated for studies of allometry. J. Evol. Biol. 30, 4–12. ( 10.1111/jeb.12986) [DOI] [PubMed] [Google Scholar]
- 37.Drès M, Mallet J. 2002. Host races in plant–feeding insects and their importance in sympatric speciation. Phil. Trans. R. Soc. Lond. B 357, 471–492. ( 10.1098/rstb.2002.1059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.García-Robledo C, Horvitz CC. 2012. Jack of all trades masters novel host plants: positive genetic correlations in specialist and generalist insect herbivores expanding their diets to novel hosts. J. Evol. Biol. 25, 38–53. ( 10.1111/j.1420-9101.2011.02401.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cenzer ML. 2016. Adaptation to an invasive host is driving the loss of a native ecotype. Evolution 70, 2296–2307. ( 10.1111/evo.13023) [DOI] [PubMed] [Google Scholar]
- 40.Schluter D. 1988. Estimating the form of natural selection on a quantitative trait. Evolution 42, 849–861. ( 10.2307/2408904) [DOI] [PubMed] [Google Scholar]
- 41.Schluter D, Nychka D. 1994. Exploring fitness surfaces. Am. Nat. 143, 597–616. ( 10.1086/285622) [DOI] [Google Scholar]
- 42.Bro-Jørgensen J. 2010. Dynamics of multiple signalling systems: animal communication in a world in flux. Trends Ecol. Evol. 25, 292–300. ( 10.1016/j.tree.2009.11.003) [DOI] [PubMed] [Google Scholar]
- 43.Miller CW, Svensson E. 2014. Sexual selection in complex environments. Annu. Rev. Entomol. 59, 427–445. ( 10.1146/annurev-ento-011613-162044) [DOI] [PubMed] [Google Scholar]
- 44.Merilä J, Kruuk LEB, Sheldon BC. 2001. Cryptic evolution in a wild bird population. Nature 412, 76–79. ( 10.1038/35083580) [DOI] [PubMed] [Google Scholar]
- 45.Wilson AJ, Pemberton JM, Pilkington JG, Coltman DW, Mifsud DV, Clutton-Brock TH, Kruuk LB.. 2006. Environmental coupling of selection and heritability limits evolution. PLoS Biol. 4, e216 ( 10.1371/journal.pbio.0040216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Husby A, Visser ME, Kruuk LE. 2011. Speeding up microevolution: the effects of increasing temperature on selection and genetic variance in a wild bird population. PLoS Biol. 9, e1000585 ( 10.1371/journal.pbio.1000585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gibson G, Dworkin I. 2004. Uncovering cryptic genetic variation. Nat. Rev. Genet. 5, 681–690. ( 10.1038/nrg1426) [DOI] [PubMed] [Google Scholar]
- 48.Cornwallis CK, Uller T. 2010. Towards an evolutionary ecology of sexual traits. Trends Ecol. Evol. 25, 145–152. ( 10.1016/j.tree.2009.09.008) [DOI] [PubMed] [Google Scholar]
- 49.Allen PE, Miller CW. 2017. Data from: Novel host plant leads to the loss of sexual dimorphism in a sexually selected male weapon. Dryad Digital Repository. ( 10.5061/dryad.5p19k) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Allen PE, Miller CW. 2017. Data from: Novel host plant leads to the loss of sexual dimorphism in a sexually selected male weapon. Dryad Digital Repository. ( 10.5061/dryad.5p19k) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The dataset supporting this article have been uploaded to Dryad Digital Repository (doi:10.5061/dryad.5p19k) [49].