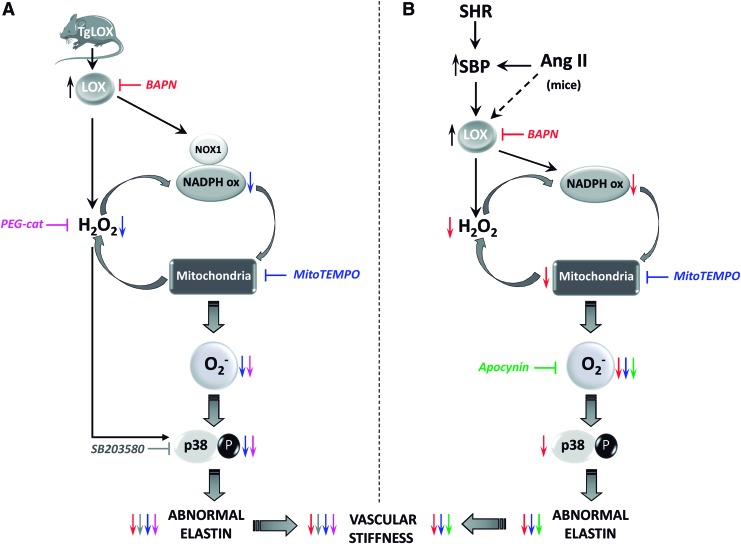
FIG. 9.
LOX induces oxidative stress that promotes p38MAPK activation, elastin remodeling, and vascular stiffness. (A) LOX transgenesis enhances vascular H2O2 levels, NADPH oxidase activity, and NOX1 expression; causes mitochondrial dysfunction; induces O2.− production; activates p38MAPK signaling; disturbs elastin structure; and increases vascular stiffness. The H2O2 scavenger PEG-catalase (in pink) and the superoxide dismutase targeted antioxidant mitoTEMPO (in blue) decrease the enhanced O2.− production and p38MAPK activation and improve elastin structure and vascular stiffness (symbolized by respective colored arrows). Accordingly, p38 MAPK inhibition by SB203580 (in grey) reduces elastin alterations and vascular stiffness (gray arrows). Further, LOX blockade with BAPN (in red) limited elastin abnormalities and vascular stiffness. (B) Vascular LOX expression is induced in animal models of hypertension (Ang II-infused mice and SHR). In both hypertensive models, LOX up-regulation partially relies on high blood pressure, although additional mechanisms could be involved (indicated with a dashed arrow). In hypertension, BAPN (in red) reduces the enhanced vascular H2O2 levels, NADPH oxidase activation, mitochondrial dysfunction, O2.− production, and/or p38MAPK activation and further ameliorates elastin abnormalities and vascular stiffness (symbolized by red arrows). Mito-TEMPO (in blue) and the antioxidant apocynin (in green) diminish oxidative stress and normalize the altered elastin structure and the increased vascular stiffness observed in hypertensive models (indicated with respective colored arrows). Note that in the vascular wall, the LOX-dependent H2O2 production would contribute to an ROS amplification mechanism involving NADPH oxidase and mitochondria. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars