Figure 4.
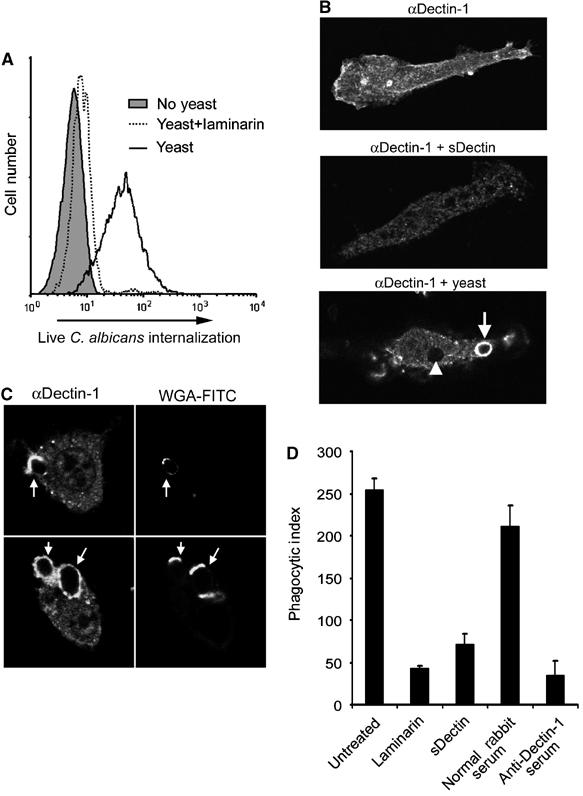
Dectin-1 mediates phagocytosis of C. albicans yeast. (A) Yeast trigger phagocytosis through Dectin-1. HEK293 cells stably expressing Dectin-1 were fed FUN-1-labeled Candida yeast. Internalization of live yeast was detected by flow cytometry. Internalization was completely inhibited by soluble β-glucan (laminarin), as indicated. (B) Dectin-1 is recruited to yeast phagosomes. Bone marrow-derived macrophages were stained with anti-Dectin-1 antibody, showing plasma membrane staining (top), which was blocked by recombinant Dectin-1 (middle). Dectin-1 was enriched on early phagosomes in macrophages fed Candida yeast (bottom, arrow), and staining was diminished on later phagosomes (arrowhead). (C) C. albicans yeast were preincubated with FITC-labeled wheat-germ agglutinin (WGA-FITC) to identify bud and birth scars, and fed to bone marrow macrophages for 10 min. Dectin-1 recruitment to phagosomes was assessed by immunofluorescence microscopy with anti-Dectin-1 antibody. (D) Bone marrow macrophages were pretreated as indicated, and fed Candida yeast for 30 min. Internalization was quantified by microscopy and is expressed as phagocytic index (particles internalized per 100 macrophages).
