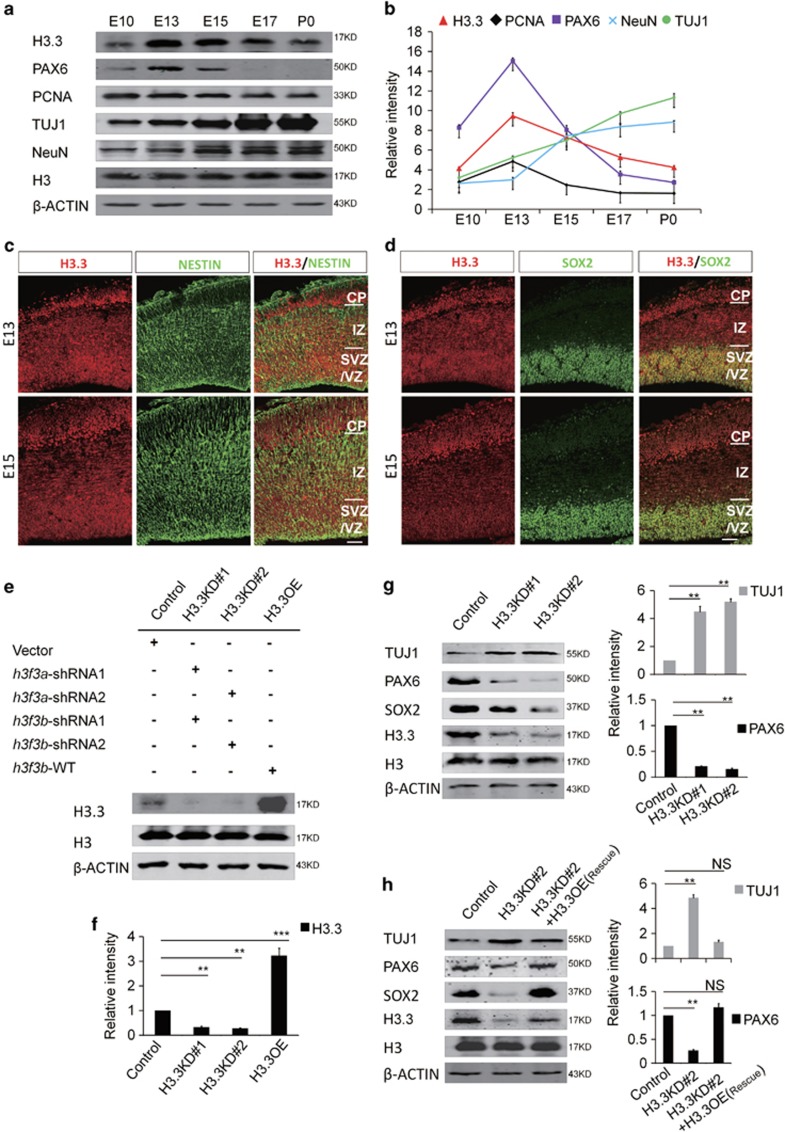
Figure 1.
Histone H3.3 Is expressed in embryonic neural progenitor cells. (a) H3.3 and different neurogenesis markers are present in cortical lysates collected at various time points (E10, E13, E15, E17, and P0). (b) A scatter diagram shows the change trend of H3.3 and different neurogenesis markers. (n=3; bar represents mean±S.E.M). (c) E13 and E15 embryonic brain sections were co-stained with anti-H3.3 and anti-NESTIN antibodies. The scale bar represents 25 μm. (d) E13 and E15 embryonic brain sections were co-stained with anti-H3.3 and anti-SOX2 antibodies. The scale bar represents 25 μm. (e,f) In the NSCs, the level of H3.3 protein is reduced by shRNA-mediated interference (H3.3KD#1: h3f3a-shRNA-1 combine with h3f3b-shRNA-1; H3.3KD#2: h3f3a-shRNA-2 combine with h3f3b-shRNA-2). H3.3 protein is increased by h3f3b-WT overexpression (n=3 independent experiments; bar represents mean±S.E.M; **P<0.01, ***P<0.001; β-ACTIN served as loading control). (g) Protein level of neural stem cell markers, including PAX6 and SOX2 are reduced in the H3.3 knockdown NSCs versus the control. However, neuronal differentiation marker TUJ1 is increased (n=3 independent experiments; bar represents mean±S.E.M; **P<0.01; β-ACTIN served as loading control). (h) Protein level changes of PAX6, SOX2, and TUJ1 caused by H3.3KD#2 (h3f3a-shRNA-2 targeting to UTR and h3f3b-shRNA-2) knockdown can be rescue by H3.3 (h3f3a) overexpression in NSCs (n=3 independent experiments; bar represents mean±S.E.M; **P<0.01; β-ACTIN served as loading control)