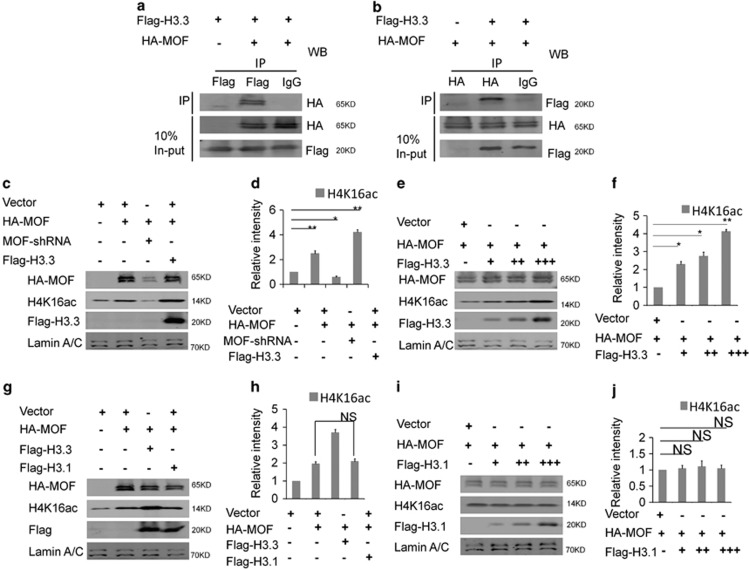
Figure 6.
H3.3 interacts with MOF directly, and H3.3/MOF promotes the acetylation of H4K16 in the collaborative role. (a) HA-MOF was immunoprecipitated by Flag-H3.3. The indicated plasmids were transfected into 293T cells. Protein lysates were divided into two parts: one for the in-put and the other for the indicated Flag-labeled magnetic beads IP. The immunoprecipitated proteins were probed with anti-HA antibodies to detect HA-MOF. (b) Flag-H3.3 was immunoprecipitated by HA-MOF. The indicated plasmids were transfected into 293 T cells. The immunoprecipitated proteins were probed with anti-Flag antibodies to detect Flag-H3.3. (c,d) H3.3 and MOF promote the acetylation of the H4K16 in the collaborative role. The indicated viruses were delivered into NSCs, and the protein level of H4K16ac was detected (n=3 independent experiments; bar represents mean±S.E.M; **P<0.01; Lamin A/C served as loading control). (e,f) H3.3 and MOF promote the acetylation of the H4K16 in the collaborative dose-response role. The indicated viruses were delivered into NSC, and the protein level of H4K16ac was detected (n=3 independent experiments; bar represents mean±S.E.M; **P<0.01; Lamin A/C served as loading control). (g–j) H3.1 could not promote the acetylation of the H4K16. The indicated viruses were delivered into NSCs, and the protein level of H4K16ac was detected (n=3 independent experiments; bar represents mean±S.E.M; Lamin A/C served as loading control)