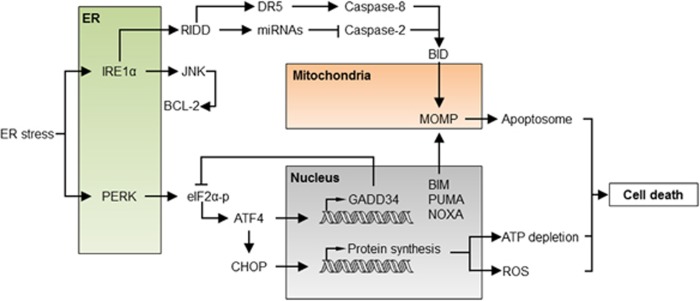
Figure 2.
The apoptosis regulatory network under ER stress. Under acute or sustained ER stress, the UPR actively promotes apoptosis through the regulation of proapoptotic proteins of the BCL-2 family, increased proteotoxicity and ROS. IRE1α activation engages the degradation of several ER-localized RNAs through RIDD, having a dual role in apoptosis regulation. Degradation of several inhibitory miRNAs leads to the activation of caspase-2, which in turn cleaves and activates the BH3-only protein BID triggering the mitochondrial pathway of apoptosis. Conversely, degradation of the DR5 mRNA results in the inhibition of caspase-8, repressing cell death. JNK is also activated downstream of IRE1α, inducing cell death through BCL-2 phosphorylation and inhibition. Activation of PERK leads to the phosphorylation of eIF2α and global protein synthesis arrest. Under these conditions, ATF4 is selectively translated and, together with CHOP, transcriptionally activates several BH3-only proteins, engaging the mitochondria. Moreover, the ATF4/CHOP complex resumes protein synthesis through the upregulation of the GADD34 phosphatase and other genes, leading to ATP depletion, ROS and finally cell death. ATF4, activating transcription factor 4; DR5, death receptor 5; GADD34, growth arrest and DNA damage-inducible protein 34; JNK, c-Jun N-terminal kinase; RIDD, regulated IRE1-dependent decay