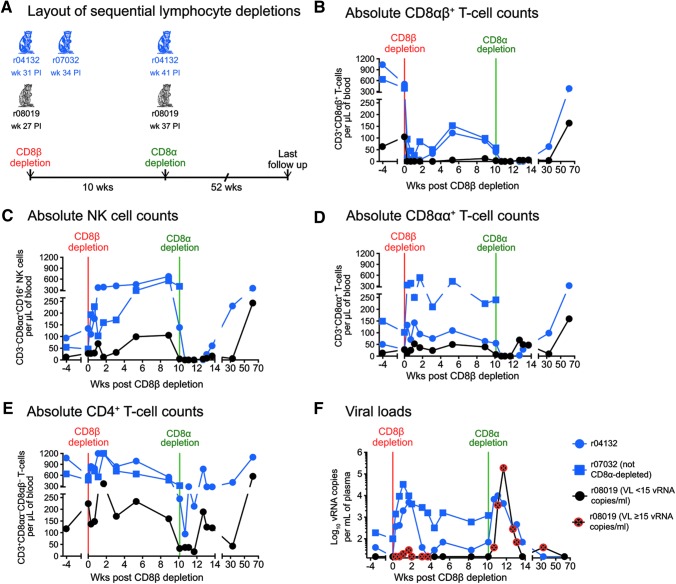
FIG. 3.
Outcome of sequential in vivo lymphocyte depletions. (A) Both Group 1 vaccinees (r04132 and r07032) and r08019 received a single infusion of 50 mg/kg of a new CD8β-specific mAb (CD8β255R1) designed to deplete CD8β-expressing cells in vivo. At the time of the CD8β depletion, r04132 was at week 31 PI; r07032 was at week 34 PI; and r08019 was at week 27 PI. Ten weeks after the CD8β depletion, CD8α+ lymphocytes in r04132 and r08019 were depleted by a single infusion of 50 mg/kg of the anti-CD8α mAb MT807R1. Due to adverse events experienced during the CD8β depletion, r07032 was not subjected to this treatment. (B–E) Absolute lymphocyte counts in blood following the CD8β and CD8α depletions. (B) CD8αβ+ T cells (live CD3+CD8αα−CD8αβ+ lymphocytes). (C) NK cells (live CD3−CD8αα+CD16+ lymphocytes). (D) CD8αα+ T cells (live CD3+CD8αα+CD8αβ−lymphocytes). (E) CD4+ T cells (considered as live CD3+CD8αα−CD8αβ−lymphocytes). (F) Log-transformed VLs after the CD8β and CD8α depletions. The time points when r08019 experienced VLs at or above the limit of reliable quantitation (15 vRNA copies/ml) are crossed in red. VLs in this animal were <15 vRNA copies/ml at all other time points shown in panel (F). mAb, monoclonal antibody.