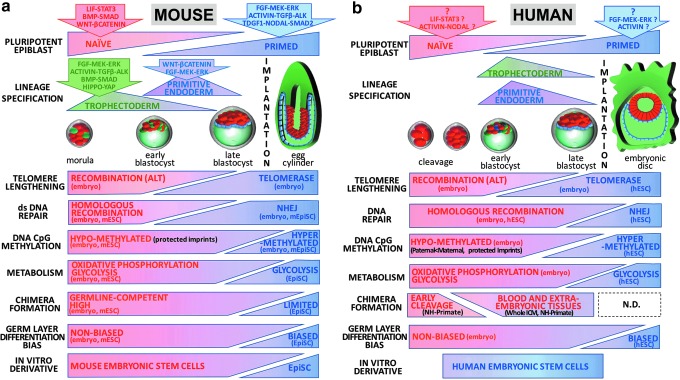
FIG. 2.
Functional phenotypes of primed and naïve pluripotent states. (a) Functional shifts in the peri-implantation mouse embryo. The mouse pluripotent epiblast progresses from a naïve ground state (red) to a primed lineage-biased state (blue) following implantation. Naïve and primed states exploit distinct signaling pathways and their transition is accompanied by the sequential specification of trophectoderm (green) and primitive endoderm (violet) lineages. Known signaling pathways directing trophectoderm and primitive endoderm are indicated. In the mouse embryo, naïve and primed states can be distinguished by differing telomere lengthening and DNA repair strategies, levels of global repressive epigenetic marks (eg, DNA CpG methylation), and usage of metabolic pathways. Both states also display nonequivalent functional pluripotencies, with only the naïve state showing capacity for germline-competent chimera formation. In contrast, postimplantation epiblast cells have a partially committed lineage bias. In vitro expansion of mouse naïve epiblast cells generates mESC lines, while the postimplantation epiblast can generate lineage-primed mEpiSC lines. Functional capacities that have been demonstrated in vivo (embryo) or using in vitro surrogates (mESC, mEpiSC) are indicated. (b) Functional shifts in the human peri-implantation embryo. Similarly to the mouse, the human pluripotent epiblast is believed to recapitulate a steady progression from a naïve preimplantation state (red) to postimplantation primed lineage-biased states (blue). The signaling pathways that are essential for human naïve and primed states remain a subject of debate and have been extrapolated from hESC or single-cell RNA sequencing of preimplantation human embryos. The progression of human pluripotency is accompanied by the specification of trophectoderm (green) and primitive endoderm (violet) lineages, although the kinetics for emergence of extraembryonic lineages diverge between both species. The human naïve and primed states can also be distinguished by differing telomere lengthening and DNA repair mechanisms, global levels of repressive epigenetic marks, and metabolic pathway usage. The chimeric contribution of the postimplantation epiblast of nonhuman primates remains undetermined. However, nonhuman primate (NH-Primate) studies indicate that chimera formation may be restricted to early cleavage embryos, with possible low engraftment capacity for later preimplantation stages demonstrated by whole ICM transfer experiments. Functional capacities that have been demonstrated in vivo (embryo) or using in vitro surrogates (hESCs) are indicated.