Figure 3.
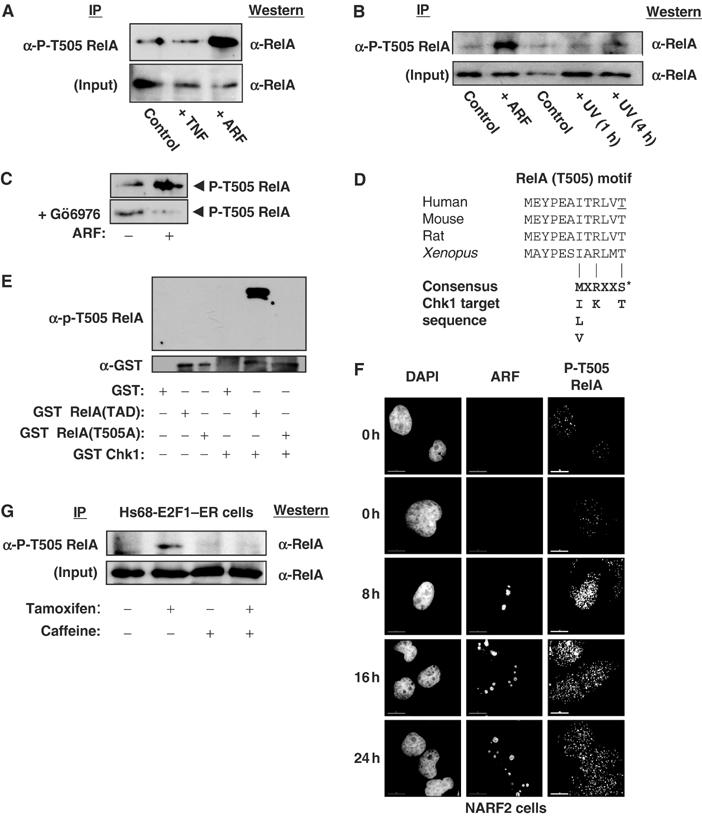
Phosphorylation of RelA at Thr505. (A) ARF induces Thr505 phosphorylation. Whole-cell lysates were prepared from NARF2 cells either treated with IPTG to induce ARF for 24 h, stimulated with TNF (10 ng/ml for 30 min) or left untreated. Phosphorylated RelA was then immunoprecipitated by incubating 150 μg of lysate with purified phospho-RelA T505A antibody. The immunoprecipitate was resolved by SDS–PAGE before Western blotting with an anti-RelA antibody (sc-372 Santa Cruz). (B) UV light does not induce Thr505 phosphorylation. The experiment was performed as in (A), except that immunoprecipitations were performed with 100 μg of nuclear protein extract. Input lanes represent 10 μg of nuclear extract. As indicated, some cells were treated with UV-C (40 J/m2) and harvested after the times shown. (C) ARF-induced Thr505 phosphorylation is prevented by the Chk1 inhibitor Gö6976. The experiment was performed as in (A), except that that indicated cells were incubated with 1 μM Gö6976 at the same time as IPTG addition. (D) Schematic diagram showing species conservation of the RelA Thr505 region and similarity to the Chk1 consensus phosphorylation sequence. The human Thr505 residue is underlined. (E) The RelA Thr505 motif is phosphorylated by Chk1 in vitro. Purified GST, GST RelA TAD (amino acids 428–551) and GST RelA TAD T505A were incubated with recombinant purified GST-Chk1 and unlabelled ATP. Proteins were then resolved by SDS–PAGE and immunoblotted with the anti-phospho RelA Thr505 antibody (upper panel). Immunoblotting with anti-GST antibody demonstrated equivalent loading of GST-RelA (TAD) and GST-RelA TAD (T505A) proteins (lower panel). (F) ARF induces nuclear phospho-Thr505-modified RelA. NARF2 cells were plated onto coverslips and fixed at the indicated times after IPTG treatment. Cells were stained with mouse anti-p14ARF and rabbit anti-P-T505 RelA antibodies. In this and subsequent experiments, cells were analysed and images were acquired using a DeltaVision microscope. (G) Induction of RelA Thr505 phosphorylation in Hs68 E2F1–ER cells. The experiment was performed as in (A), except that immunoprecipitations were performed with 300 μg of whole-cell lysates from Hs68 E2F1–ER cells. Input lanes represent 30 μg of extract. As indicated, cells were treated with Tamoxifen and 2 mM caffeine and harvested 24 h later.
