Abstract
Using a novel magnetic field bioreactor, this work evaluated the chondrogenesis of scaffold-free human mesenchymal stem cell sheets in response to static and variable magnetic fields, as well as mechanical stimulation via 4.4 µm magnetic particles. Neither static nor variable magnetic fields generated by 1.44 – 1.45 T permanent magnets affected cartilage formation. Notably, magnetic field-induced mechanical stimulation by magnetic particles, which applied forces to the cells and ECM statically (4.39 pN) or cyclically (1.06 – 63.6 pN; 16.7 mHz), also did not affect cartilage formation.
Graphical abstract
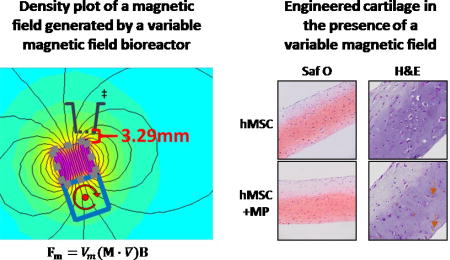
Neither magnetic fields (variable or static) nor magnetically-induced mechanical stimulation via magnetic microparticles influenced hMSC chondrogenesis in tissue engineered cartilage sheets.
Introduction
Mechanical stimuli play an important role in directing cellular behavior and determining extracellular matrix (ECM) composition and architecture in many tissues1–3. Typically, the role of mechanical stimuli on tissue regeneration is investigated on the macroscopic scale where external stimuli like hydrostatic, compressive, tensile and/or shear stress are applied to bulk tissue. Studies have shown that cartilage-like properties of tissue engineered constructs can be enhanced by bioreactor-induced loading2, 4, 5. Mechanobiology on the microscale has also been investigated, often using magnetic particles6. For example, cell-ECM interactions have been studied by perturbing magnetic particles bound to integrins7, which are transmembrane proteins that link the ECM to the cytoskeleton and transmit forces that regulate cellular activities8. However, magnetic particle-driven mechanostimulation for orthopedic applications has only been pursued for bone tissue formation9–12. In monolayer culture of osteoblasts with 4–4.5 µm arginine-glycine-aspartic acid (RGD)-coated magnetic particles, which were initially bound to the surface of the cells and later internalized, expression of bone matrix specific genes9 and levels of intracellular calcium10 were enhanced by applying a variable (1 Hz) or static magnetic field, which generated a force of ~3 pN per particle10. In 3D in vitro culture and in vivo implantation, magnetic nanoparticles targeted to stretch activated potassium channels (TREK-1) or integrins on human bone marrow stromal cells enhanced their osteogenic differentiation when stimulated with a force of 1–100 pN/particle at 1 Hz12. These studies demonstrate the promise of microscale mechanostimulation of cells for controlling cell differentiation and metabolic activity.
While application of a magnetic field without the incorporation of a magnetically responsive material into tissue engineered constructs has been reported to enhance chondrogenesis13–15, mechanostimulation via magnetic particles has not been evaluated on its ability to improve cartilage formation. In this work, the ability of static and variable magnetic fields to enhance chondrogenesis of scaffold-free human mesenchymal stem cell (hMSC) sheets undergoing chondrogenesis16 without and with incorporated magnetic particles, which exert forces on cells and/or surrounding ECM in response to the gradients in the magnetic field, was investigated in a custom-made magnetic field bioreactor.
Methods
hMSC isolation and culture
hMSCs were isolated from purchased whole bone marrow (Case Comprehensive Cancer Center Hematopoietic Biorepository and Cellular Therapy Core; harvested under the approval of University Hospitals of Cleveland Institutional Review Board)17 and were culture-expanded in Dulbecco’s Modified Eagle’s Medium - low glucose (DMEM-LG; Sigma-Aldrich) containing 10% pre-screened fetal bovine serum (Gibco Qualified FBS; Life Technologies or Sigma Premium FBS; Sigma-Aldrich)18 and 10 ng/ml fibroblast growth factor-2 (FGF-2, R&D Systems). Passage 3 cells from 2 different donors (donor 1 was a 27-year-old female; donor 2 was 28-year-old male) were used in this study. Passage 2 cells from donor 1 were used in the Electronic Supplementary Information (ESI) data.
Magnetic hMSC sheet preparation
Serum proteins were non-specifically adsorbed onto the surface of magnetic particles (“MP”; DynaBeads M-450, ThermoFisher Scientific) by incubating 100 µl particle solution (4×108 MPs/ml) with 900 µl FBS at room temperature overnight on a rotisserie rotator (Labquake, Barnstead Thermolyne) to facilitate particle incorporation into the cell sheet. Cell culture inserts (3.0 µm pore, 6.5 mm diameter polycarbonate transwells, Corning) were incubated with 450 µl DMEM-LG with 10% FBS in the wells of a 24-well plate for 2 hours. Next, 0.6×106 hMSCs were mixed with 0.6×106 MPs (“hMSC + MP”) and seeded onto the insert in 100 µl chondrogenic media consisting of Dulbecco's Modified Eagle's Medium - high glucose (DMEM-HG; Sigma-Aldrich), 1% ITS+ Premix (Corning), 10−7 M dexamethasone (MP Biomedicals), 1 mM sodium pyruvate (HyClone Laboratories), 100 mM non-essential amino acids (Lonza Group), 37.5 mg/ml ascorbic acid-2-phosphate (Wako Chemicals USA) and 10 ng/ml transforming growth factor beta 1 (TGF-β1, PeproTech). Lastly, an additional 450 µl of chondrogenic media was added to the plate well. Control sheets without MPs (“hMSC”) were prepared in the same manner. hMSC ± MP were cultured in 1 ml chondrogenic medium replaced every 2 days for 3 weeks.
Application of magnetic field
All hMSC sheets with and without MPs were cultured for the first 3 days without exposure to a magnetic field. Control sheets were cultured without a magnetic field for the duration of the experiment.
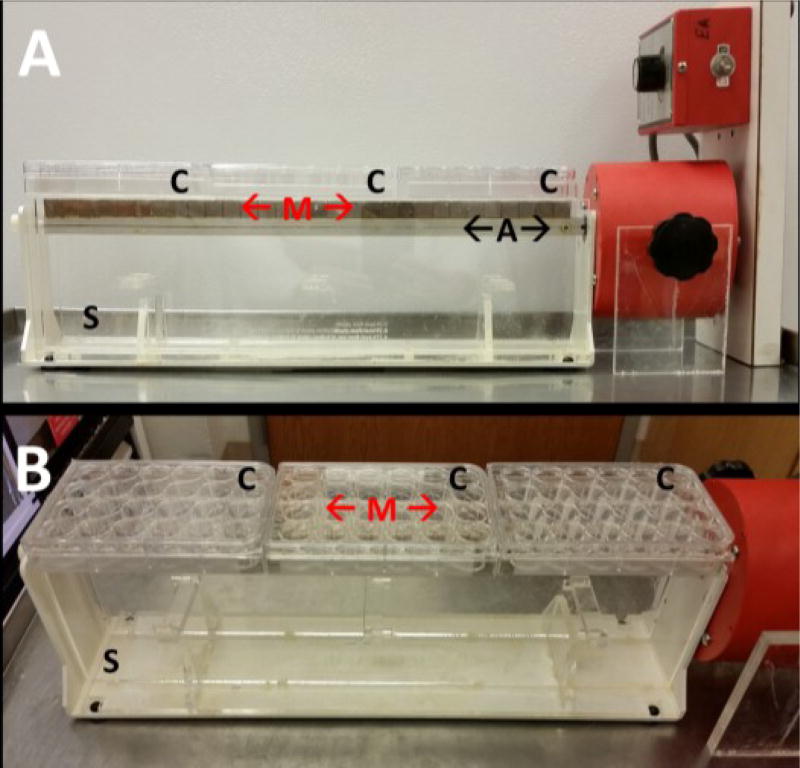
The variable magnetic field (VMF) bioreactor consisted of 30 rare earth N52 permanent magnets (1.27 cm cubes, 1.45 T; CMS Magnetics) glued in a continuous strip onto an aluminum axle of an adapted variable speed mixer (Model 1, Pelco Infiltron) with alternating pole orientation (north-south-north etc.) to achieve maximum magnetic field gradients. Multiwell plates containing hMSC ± MP sheets in the 2 middle rows were placed above the rotating magnet bar on a custom-made polycarbonate support platform (Fig 1), with the path of rotation of the magnets being wider than the middle 2 rows of the plate. Throughout magnet rotation, hMSC sheets were 3.29 – 29.72 mm away from the magnet, which generated oscillating forces of 1.06 – 63.6 pN on each magnetic particle (see ESI for calculations of the force on a MP due to the magnetic field). For all VMF conditions, the magnetized bar rotated at 1 rpm (16.7 mHz) for 1 hr to create a VMF followed by no rotation for 1 hr continuously. Starting on day 3 of culture, hMSC ± MP sheets were exposed to VMF for 1 hr/day (1 hr at 1 rpm, 1 hr static), 3 hrs/day ([1 hr at 1 rpm, 1 hr static] × 3) or 12 hrs/day [1 hr at 1 rpm, 1 hr static] × 12], 5 days/week for 3 weeks. The distance between the sheets and the magnet during the static period was variable.
Fig 1.
(A) Side and (B) angled views of the variable magnetic field bioreactor comprised of permanent magnets (“M”) affixed to a rotating aluminium axle ("A"). Multiwell culture plates ("C”) containing engineered tissues were suspended above the rotating magnets on a custom polycarbonate support platform ("S").
Static magnetic field (SMF) was applied by placing a multiwell plate with hMSC ± MP sheets directly on top of N52 permanent magnets (5.08 cm × 5.08 cm × 1.27 cm (LxWxH) block, 1.44 T; CMS Magnetics) without the support platform. The distance between the sheets and the magnet was 2.28 mm resulting in 4.39 pN of force on each magnetic particle (see ESI). hMSC sheets with and without MPs were exposed to SMF 24 hr/day, 5 days/week for 3 weeks.
Analysis of harvested hMSC sheets
hMSC sheets with and without magnetic particles (N=3) were evaluated for glycosaminoglycan (GAG; a major component of hyaline cartilage) content via dimethylmethylene blue assay (DMMB; Sigma-Aldrich)19 and DNA content via PicoGreen assay (Invitrogen)20. Data was analyzed via one-way ANOVA with Tukey's post hoc tests (p < 0.05; InStat 3.06, GraphPad Software Inc.). Data is represented as mean ± standard deviation.
Harvested sheets were also fixed in 10% neutral buffered formalin, paraffin embedded and sectioned (5 µm). Tissues (N=3) were stained with Hematoxylin (Fisher HealthCare) & Eosin (Richard-Allan Scientific) and Safranin O (Acros Organics) for sulfated GAG content with a Fast Green (Fisher Scientific) counterstain21.
Results and Discussion
High-density hMSC sheets with and without magnetic particles were exposed to a static magnetic field and a variable magnetic field for a range of durations to evaluate the effects of a magnetic field itself and magnetically-induced mechanical perturbations of embedded magnetic particles within cellular sheets on chondrogenesis. hMSCs derived from 2 different donors were compared to elucidate donor-dependent differences in chondrogenic response. After 3 weeks of cartilage formation, sheets were harvested and analyzed biochemically and histologically.
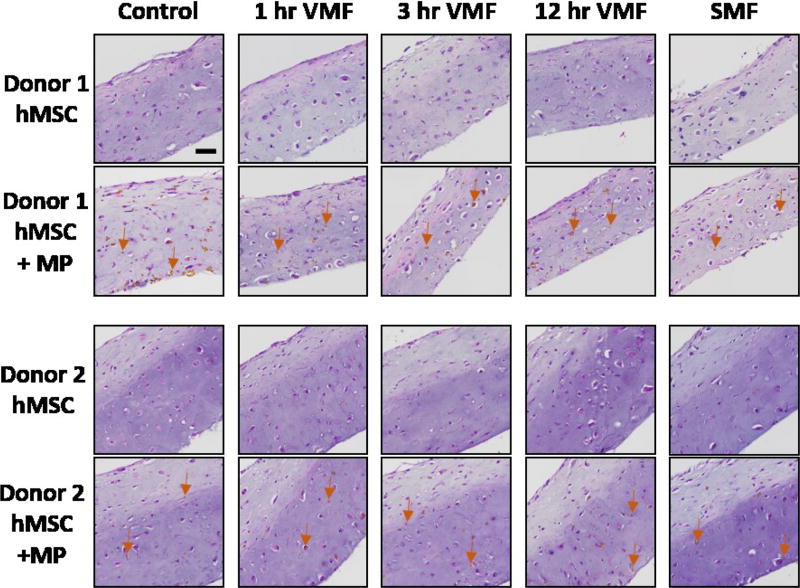
Magnetic particles were distributed throughout the thickness of 3-week-old sheets with a slight accumulation of the MPs on the lower portion of all sheets (Fig 2). This effect was exacerbated in sheets exposed to a magnetic field compared to control sheets. As a result, mechanical forces application may not have been homogeneously applied throughout the sheet. The slight MP accumulation on the bottom of sheets exposed to a magnetic field was likely due to the magnetic force pulling the MPs toward the magnet under the sheets. The cyclical maximum and minimum forces on each particle were calculated to be 63.6 pN and 1.06 pN, respectively, in the VMF groups, and 4.39 pN in the SMF condition (see ESI, Fig S.1–S.4), which are of the same order of magnitude as previously reported to enhance osteogenesis of hMSCs12. Throughout the 16.7 mHz cyclical stimulation, it is possible that when the magnets were closest to the sheets, applying maximum forces, MPs were pulled and displaced towards the magnets, and when the magnets were farthest from the sheets, creating minimum forces, some MPs may have been able to recoil to some degree due to the viscoelastic nature of neocartilage22. Another reason for the uneven MP distribution could be deposition of new ECM. As cartilaginous ECM accumulated and sheet thickness increased, magnetic particles may not have become evenly mixed with the newly synthesized ECM. In contrast to osteoblasts in 2D culture which had almost complete particle internalization by 48 hours10, hMSCs and/or differentiated chondrocytes in this 3D culture did not internalize the 4.4 µm MPs as readily as some of the MPs were in the bulk ECM while some were internalized. However, both internalized and integrin-attached particles have been reported to induce cellular responses10, so it is possible that cytosolic and extracellular MPs would also be able to affect cellular activity of hMSCs undergoing chondrogenic differentiation in this system.
Fig 2.
H&E stained hMSC sheets with and without magnetic particles (MP) from 2 donors. Control sheets were never exposed to a magnetic field. Stimulated sheets were exposed to a variable magnetic field (VMF) for 1 hour, 3 hours or 12 hours daily or a continuous static magnetic field (SMF) 5 days/week for 3 weeks. Arrows denote MPs. All images are at the same magnification. Scale bar is 50 µm.
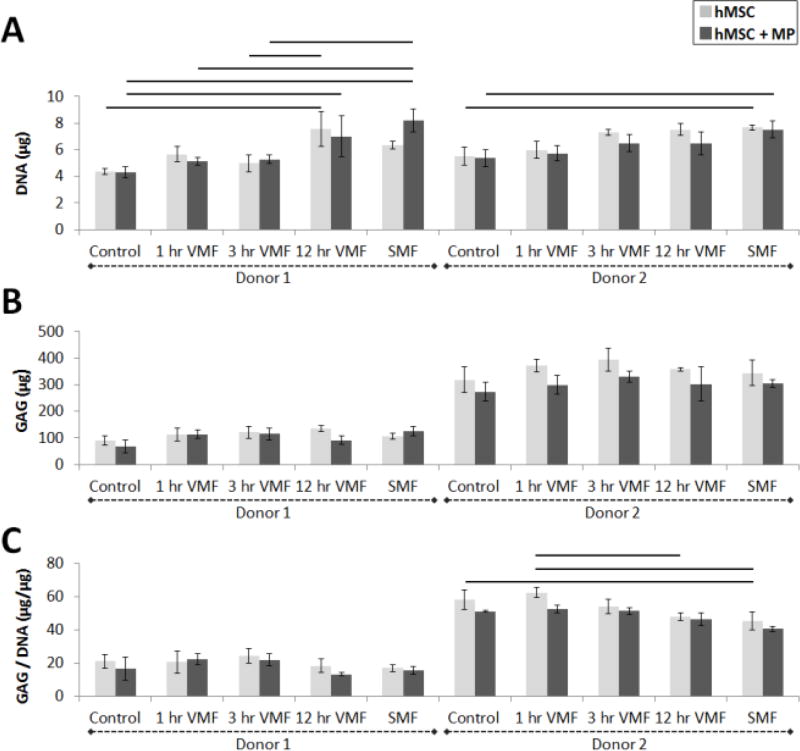
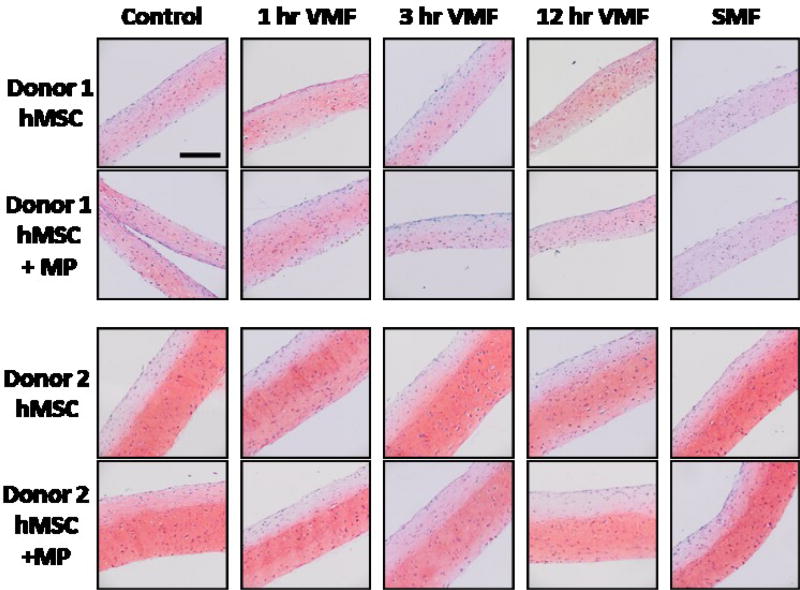
The incorporation of magnetic particles did not have an effect on cell number or GAG accumulation in groups that received the same magnetic field treatment. Within each donor, there were no significant differences in GAG, DNA or GAG normalized to DNA contents between sheets with and without particles for each stimulation regime (Fig 3A–B). Replicate independent experiments with similar magnetic field stimulation regimes had similar results (Fig S.5A–B, Fig S.6A–B). Histologically, the intensity and distribution of Safranin O staining and tissue morphology were similar in sheets with and without magnetic particles (Fig 4). Our findings are supported by previous reports where the presence of magnetic particles during monolayer hMSC culture did not induce cell death12 or have an effect on hMSC differentiation23.
Fig 3.
(A) DNA, (B) GAG and (C) GAG normalized to DNA of hMSC sheets with (dark gray) and without (light gray) magnetic particles (MP) from 2 donors. Control sheets were never exposed to a magnetic field. Stimulated sheets were exposed to a variable magnetic field (VMF) for 1 hour, 3 hours or 12 hours daily or a continuous static magnetic field (SMF) 5 days/week for 3 weeks. Lines denote statistical significance of p<0.05.
Fig 4.
Safranin O staining for GAG in hMSC sheets with and without magnetic particles (MP) from 2 donors with a Fast Green counterstain. Control sheets were never exposed to a magnetic field. Stimulated sheets were exposed to a variable magnetic field (VMF) for 1 hour, 3 hours or 12 hours daily or a continuous static magnetic field (SMF) 5 days/week for 3 weeks. All images are at the same magnification. Scale bar is 200 µm.
Exposure to a magnetic field may increase cell proliferation or reduce cell death but results were inconsistent. In general, the longer hMSC ± MP sheets were exposed to a magnetic field (variable or static), the DNA content of tissue engineered sheets increased for both donors (Fig 4A). The increase in DNA was significant for some of the groups. However, similar experiments performed with donor 2 did not corroborate these findings as DNA content was not affected by exposure to a magnetic field (Fig S.5A, Fig S.6A). Inconsistent results have also been reported in the literature (see review24), and may be a result of differences in cells type and/or parameters of applied magnetic field. Some studies show that application of a strong static magnetic field by itself (without the use of MPs) may reduce cell numbers: a 3 T magnetic field from an MRI machine induced apoptosis of chondrocytes cultured in monolayer25 and an averaged 0.618 mT field decreased proliferation of monolayer-cultured osteoblasts26. Other studies demonstrated the opposite: a 0.6 T static magnetic field increased viability of monolayer-cultured chondrocytes27 and 1 T pulsed magnetic field (a series of 30 single 5 ms duration (200 Hz) pulses with 5 s in between pulses) did not affect viability of human adipose-derived stem cell pellets cultured in chondrogenic media14. In addition, other groups do not report the effect of magnetic field on DNA content in their systems10, 13, 23.
Magnetic field stimulation of hMSC-only and magnetic particle-laden hMSC sheets did not enhance chondrogenesis. Quantified total GAG content was not statistically significant between different stimulation groups (Fig 3B). These findings were supported by Safranin O staining for GAG which was similar for all stimulation regimes in both donors (Fig 4). Some statistically significant differences between magnetic field stimulation regimes were evident in GAG normalized to DNA data, which showed that increased exposure to a magnetic field (12 hr VMF or SMF) may decrease GAG/DNA in hMSC sheets without MPs compared to 1 hr VMF or unstimulated control sheets (Fig 3C). In a follow-up experiment, hMSCs + MP sheets exposed to 8 hr VMF (0.39 T magnet) resulted in decreased GAG/DNA compared to unstimulated hMSCs + MP sheets (Fig S.5C). However, in yet another experiment, there were no significant differences in GAG/DNA between different stimulation regimes (Fig S.6C). Taken all together, our findings suggest that static and variable magnetic fields from 1.44–1.45 T magnets do not meaningfully affect chondrogenesis of hMSC sheets regardless of whether or not magnetic particles are incorporated into the tissues based on quantified GAG and GAG/DNA contents and histological staining. The lack of enhanced cartilage formation in particle-loaded sheets could be due to, for example, inadequate activation of mechanoreceptors such as stretch-activated ion channels or integrins, which were previously reported to be stimulated via targeted nanoparticles during osteogenic differentiation of MSCs12. To test this, follow-up studies could evaluate the effects receptor-targeted particles, incorporation of variable amounts of particles and/or additional stimulation regimes.
While there have not been prior studies examining the effects of magnetic fields on chondrogenesis of hMSC pellets or sheets with incorporated MPs, these results are different from previously published findings on the effects of magnetic fields without MPs on chondrogenesis. A static magnetic field (0.4 T magnet) increased GAG/DNA in hMSC pellets undergoing chondrogenesis in vitro compared to non-stimulated groups13. A pulsed electromagnetic magnetic field (1 T; 30 single pulses each 5 ms in duration (200 Hz) with 5 s in between pulses) elevated chondrogenic gene expression and GAG/DNA of human adipose-derived stem cells in monolayer and pellet cultures in growth medium to similar levels as cells grown in chondrogenic medium14. In vivo, an estimated 8 mT static field induced healing of osteochondral defects in rabbits compared to sham-operated groups15 and a 1.6 mT pulsed electromagnetic field (4.5 ms duration (222 mHz) single pulse repeated at 15 bursts/s, 8 hr/day) accelerated chondrogenesis during endochondral ossification28. These few studies illustrate that magnetic fields by themselves may influence chondrogenesis in specific circumstances, although the mechanisms behind its actions are poorly understood5, 24. Potential reasons why enhanced chondrogenesis was not observed in our system compared to these reports may be differences in cell type, and magnetic field gradient magnitudes, frequencies and durations.
Conclusions
This work evaluated the effects of (1) static and variable magnetic fields and (2) mechanical stimulation applied via magnetic particles on chondrogenesis within scaffold-free, high-density hMSC sheets in a novel magnetic field bioreactor. Magnetic field exposure by itself did not enhance chondrogenesis in cell-only sheets. Interestingly, even when magnetically responsive particles were incorporated within hMSC sheets, mechanical force application also did not improve cartilage formation. In light of our findings, additional studies may be necessary to validate previously published reports of enhanced chondrogenesis in response to magnetic fields, to determine the specific conditions necessary for the observed responses and to elucidate the cellular mechanisms leading to enhanced chondrogenesis observed.
Supplementary Material
Acknowledgments
The authors would like to thank Amad Awadallah for histological services. This work was funded by National Institutes of Health (R01AR063194 (EA) and T32AR007505 (ADD)) and the Medtronic Foundation (ADD).
Footnotes
Electronic Supplementary Information (ESI) available: Modeling of forces on a magnetic particle; Data from supplementary experiments of hMSC sheets without and with magnetic particles stimulated by magnetic fields. See DOI: 10.1039/x0xx00000x
References
- 1.McCullen SD, Haslauer CM, Loboa EG. J. Biomech. 2010;43:119–127. doi: 10.1016/j.jbiomech.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Grad S, Eglin D, Alini M, Stoddart MJ. Clin. Orthop. Relat. Res. 2011;469:2764–2772. doi: 10.1007/s11999-011-1819-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilak F, Butler DL, Goldstein SA, Baaijens FP. J. Biomech. 2014;47:1933–1940. doi: 10.1016/j.jbiomech.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz RM, Bader A. Eur. Biophys. J. 2007;36:539–568. doi: 10.1007/s00249-007-0139-1. [DOI] [PubMed] [Google Scholar]
- 5.Brady MA, Waldman SD, Ethier CR. Tissue Eng Part B Rev. 2015;21:1–19. doi: 10.1089/ten.TEB.2013.0757. [DOI] [PubMed] [Google Scholar]
- 6.Janmey PA, McCulloch CA. Annu Rev Biomed Eng. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- 7.Overby DR, Matthews BD, Alsberg E, Ingber DE. Acta Biomater. 2005;1:295–303. doi: 10.1016/j.actbio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Wang N, Butler JP, Ingber DE. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 9.Cartmell SH, Dobson J, Verschueren SB, El Haj AJ. IEEE Trans Nanobioscience. 2002;1:92–97. doi: 10.1109/tnb.2002.806945. [DOI] [PubMed] [Google Scholar]
- 10.Hughes S, Dobson J, El Haj AJ. J. Biomech. 2007;40(Suppl 1):S96–104. doi: 10.1016/j.jbiomech.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Dobson J, Cartmell SH, Keramane A, El Haj AJ. IEEE Trans Nanobioscience. 2006;5:173–177. doi: 10.1109/tnb.2006.880823. [DOI] [PubMed] [Google Scholar]
- 12.Kanczler JM, Sura HS, Magnay J, Green D, Oreffo RO, Dobson JP, El Haj AJ. Tissue Eng Part A. 2010;16:3241–3250. doi: 10.1089/ten.TEA.2009.0638. [DOI] [PubMed] [Google Scholar]
- 13.Amin HD, Brady MA, St-Pierre JP, Stevens MM, Overby DR, Ethier CR. Tissue Eng Part A. 2014;20:1612–1620. doi: 10.1089/ten.tea.2013.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CH, Lin YS, Fu YC, Wang CK, Wu SC, Wang GJ, Eswaramoorthy R, Wang YH, Wang CZ, Wang YH, Lin SY, Chang JK, Ho ML. J Appl Physiol (1985) 2013;114:647–655. doi: 10.1152/japplphysiol.01216.2012. [DOI] [PubMed] [Google Scholar]
- 15.Jaberi FM, Keshtgar S, Tavakkoli A, Pishva E, Geramizadeh B, Tanideh N, Jaberi MM. Arch. Med. Res. 2011;42:268–273. doi: 10.1016/j.arcmed.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Solorio LD, Vieregge EL, Dhami CD, Dang PN, Alsberg E. J. Control. Release. 2012;158:224–232. doi: 10.1016/j.jconrel.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Bone. 1992;13:81–88. doi: 10.1016/8756-3282(92)90364-3. [DOI] [PubMed] [Google Scholar]
- 18.Lennon DP, Haynesworth SE, Bruder SP, Jaiswal N, Caplan AI. In Vitro Cellular & Developmental Biology-Animal. 1996;32:602–611. [Google Scholar]
- 19.Farndale RW, Buttle DJ, Barrett AJ. Biochim. Biophys. Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 20.McGowan KB, Kurtis MS, Lottman LM, Watson D, Sah RL. Osteoarthritis Cartilage. 2002;10:580–587. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- 21.Dikina AD, Strobel HA, Lai BP, Rolle MW, Alsberg E. Biomaterials. 2015;52:452–462. doi: 10.1016/j.biomaterials.2015.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park S, Ateshian GA. J. Biomech. Eng. 2006;128:623–630. doi: 10.1115/1.2206201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu B, El Haj AJ, Dobson J. Int. J. Mol. Sci. 2013;14:19276–19293. doi: 10.3390/ijms140919276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dini L, Abbro L. Micron. 2005;36:195–217. doi: 10.1016/j.micron.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh CH, Lee MC, Tsai-Wu JJ, Chen MH, Lee HS, Chiang H, Herbert Wu CH, Jiang CC. Osteoarthritis Cartilage. 2008;16:343–351. doi: 10.1016/j.joca.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Cohly HH, Abraham GE, 3rd, Ndebele K, Jenkins JJ, Thompson J, Angel MF. Biomed. Sci. Instrum. 2003;39:454–459. [PubMed] [Google Scholar]
- 27.Stolfa S, Skorvanek M, Stolfa P, Rosocha J, Vasko G, Sabo J. Physiol. Res. 2007;56(Suppl 1):S45–49. doi: 10.33549/physiolres.931301. [DOI] [PubMed] [Google Scholar]
- 28.Ciombor DM, Lester G, Aaron RK, Neame P, Caterson B. J. Orthop. Res. 2002;20:40–50. doi: 10.1016/S0736-0266(01)00071-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.