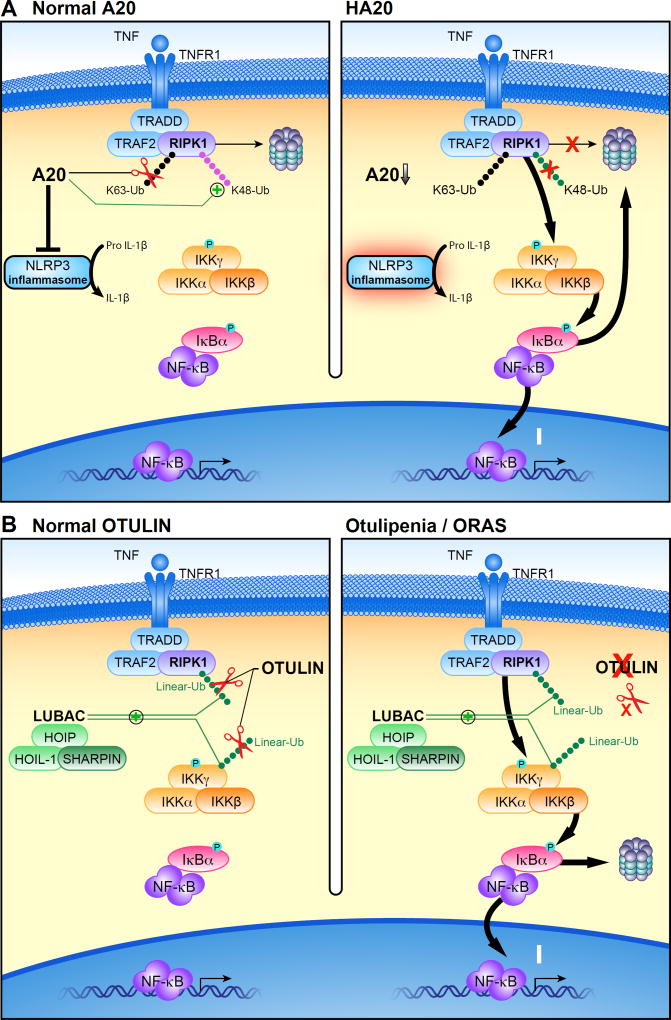
Figure 1. Proposed mechanisms of pathogenesis in deubiquitinase deficiencies.
(A) After binding TNF, a signaling complex is recruited to the TNF receptor. The addition of lysine 63 (K63)-linked ubiquitin (Ub) chains to RIPK1 stabilizes its signaling complex and leads to phosphorylation of the IKK complex, degradation of IκBα, and translocation of the NF-κB heterodimer into the nucleus. Under normal conditions (left), A20 removes K63-linked Ub chains from the RIPK1 complex and instead conjugates its constituent proteins with lysine 48 (K48)-linked Ub chains to mark them for proteasomal degradation. In haploinsufficiency of A20 (HA20), heterozygous loss of function of A20 results in the impaired suppressive function of A20, leading to excessive activation of NFκB signaling (right).
(B) The linear ubiquitin assembly complex (LUBAC) conjugates methionine 1-linked ubiquitin (linear-Ub) to targets including NEMO and RIPK1, which potentiates NF-κB signaling. OTULIN restricts this signaling by hydrolyzing linear-Ub on LUBAC and its target proteins (left). In otulipenia/OTULIN-related autoinflammatory syndrome (ORAS), biallelic loss of function of OTULIN results in loss of this suppressive function, leading to accentuated activation of NF-κB signaling (right). For the sake of simplicity, ubiquitination on TRAF2 and TNFR1 are not shown in these figures.