Abstract
Breast cancer is a major cause of cancer-related death. TRAIL has been of interest as a cancer therapeutic, but only a subset of triple negative breast cancers (TNBC) is sensitive to TRAIL. The small molecule ONC201 induces expression of TRAIL and its receptor DR5. ONC201 has entered clinical trials in advanced cancers. Here we show that ONC201 is efficacious against both TNBC and non-TNBC cells (n=13). A subset of TNBC and non-TNBC cells succumb to ONC201-induced cell death. In 2/8 TNBC cell lines, ONC201 treatment induces caspase-8 cleavage and cell death that is blocked by TRAIL-neutralizing antibody RIK2. The pro-apoptotic effect of ONC201 translates to in vivo efficacy in the MDA-MB-468 xenograft model. In most TNBC lines tested (6/8) ONC201 has an anti-proliferative effect but does not induce apoptosis. ONC201 decreases cyclin D1 expression and causes an accumulation of cells in the G1 phase of the cell cycle. pRb expression is associated with sensitivity to the anti-proliferative effects of ONC201, and the compound synergizes with taxanes in less sensitive cells. All non-TNBC cells (n=5) are growth inhibited following ONC201 treatment, and unlike what has been observed with TRAIL, a subset (n=2) show PARP cleavage. In these cells, cell death induced by ONC201 is TRAIL-independent. Our data demonstrate that ONC201 has potent anti-proliferative and pro-apoptotic effects in a broad range of breast cancer subtypes, through TRAIL-dependent and TRAIL-independent mechanisms. These findings develop a pre-clinical rationale for developing ONC201 as a single agent and/or in combination with approved therapies in breast cancer.
Keywords: ONC201, TRAIL, breast cancer, anti-proliferative, integrated stress response
Introduction
ONC201 is a member of a new class of anti-cancer compounds known as the imipridones [1]. ONC201 was originally identified as a p53-independent transcriptional inducer of TNF-related apoptosis inducing ligand (TRAIL) [2,3]. TRAIL is a protein that binds to death receptors DR4 and DR5, inducing cell death in tumor cells but not normal cells [4]. While anti-DR5 agonistic antibodies have shown limited activity in triple negative breast cancer (TNBC) [3], most breast cancers are resistant to TRAIL and TRAIL based therapies [5]. Identified as a TRAIL-inducing compound, ONC201 (originally called TIC10) represents a novel type of TRAIL pathway activating therapy. The mechanism by which ONC201 induces the TRAIL gene involves inactivation of pro-survival kinases Akt and ERK, leading to decreased phosphorylation of transcription factor FOXO3a [3]. Dephosphorylated FOXO3a translocates to the nucleus to bind and activate its target gene TRAIL. In addition to increasing surface expression of TRAIL, ONC201 activates a PERK-independent integrated stress response (ISR) [6], leading to increased transcription of TRAIL receptor DR5. Preclinical studies involving ONC201 have shown that it has effects on chemotherapy-resistant cancer stem cells [7], a wide therapeutic index [8], and anti-proliferative and pro-apoptotic effects in a range of tumor types [6,9,10]. The compound has completed its first-in-human clinical trial [11] and is currently being tested in multiple phase II studies.
Breast cancer is currently the most commonly diagnosed cancer in women in the United States, and the second leading cause of cancer-related death. The SEER program estimates that in 2016 there were 246,660 women diagnosed with breast cancer and 40,450 deaths. Gene expression profiling divides breast cancers into molecular subtypes [12]. These subtypes correlate with the presence or absence of different receptors, including the estrogen receptor (ER), progesterone receptor (PR), and receptor tyrosine kinase HER2/ERBB2. HER2+ breast cancers express high levels of HER2, hormone receptor positive breast cancers express high levels of ER or PR, and the highly aggressive triple negative breast cancers (TNBC) lack expression of all three receptors. TNBC tumors can be further divided into 6 subtypes on the basis of gene expression profiling [13]. These include two basal-like (BL-1 and BL-2), mesenchymal-like, mesenchymal stem-like, immunomodulatory, and luminal androgen receptor subtypes. Standard of care treatments for breast cancer are subtype-specific and involve chemotherapy, endocrine targeting therapies, and radiation. All of these therapies have significant adverse effects. The majority of breast cancer cell lines have been shown to have intrinsic resistance TRAIL-mediated apoptosis, with the exception of a subset of TNBCs [14]. Different mechanisms of TRAIL resistance have been characterized in breast cancers, including low surface expression of death receptors [15] and high levels of IAP and Bcl-2 family anti-apoptotic proteins [16]. ONC201 has been previously shown to affect these resistance mechanisms, increasing cell surface DR5 expression [4] and decreasing expression of IAP and Bcl-2 family anti-apoptotic proteins [5]. These observations led to a hypothesis that ONC201 may be efficacious in both TNBC and non-TNBC cells. Here, we report that ONC201 shows efficacy in TNBC and non-TNBC cells through both TRAIL-dependent and TRAIL-independent mechanisms.
Materials and Methods
Cell culture and reagents
Breast cancer cell lines were obtained from the Fox Chase Cancer Center cell culture facility in 2016 and confirmed to be mycoplasma free using PCR testing methods. Cell lines were authenticated in the last 6 months using Short Tandem Repeat profiling (IDEXX BioResearch). Cell lines were cultured in media specified by the ATCC, supplemented with 10% fetal bovine serum and 1% penicillin streptomycin. Cells were passaged minimally between time of thawing and time of use in experiments. Paclitaxel and docetaxel were obtained from Selleckchem and reconstituted in DMSO. ONC201 was supplied by Oncoceutics, Inc and reconstituted in DMSO.
Cell viability assays
Cell were seeded in 96-well black bottom plates (5 × 103 cells/well) and allowed to adhere for 24 hours before the addition of DMSO vehicle, ONC201, docetaxel, paclitaxel, or TRAIL. Viability was assessed using the CellTiter-Glo luminescent cell viability assay (Promega) per the manufacturer’s instructions. Dose-response curves were constructed using GraphPad Prism software.
Apoptosis and cell cycle assays
Annexin-PI staining was performed to quantitate apoptotic cells using the Dead Cell Apoptosis Kit with Annexin V Alexa Fluor™ 488 and Propidium Iodide kit (Thermo Fisher Scientific) per the manufacturer’s instructions. RIK2 TRAIL neutralizing antibody (Santa Cruz Biotechnology) was used to a final concentration of 1 μg/mL. BrdU-PI staining was performed to assess the effects of ONC201 on proliferation and cell cycle profile. BrdU was added to culture media to a final concentration of 10 μM. 30 minutes later, floating and adherent cells were collected and fixed in 70% ethanol. Cells were washed and stained with a mouse anti-BrdU antibody (BD Biosciences) for 30 minutes at room temperature. Cells were washed and stained with an anti-mouse Alexa Fluor 488 secondary antibody (Invitrogen) for 30 minutes at room temperature. Cells were washed and resuspended in a propidium iodide staining solution (5 μg/mL propidium iodide and 200 μg/mL RNase in PBS). Flow cytometry data was collected using an Elite Epics flow cytometer (Coulter-Beckman). FlowJo™ software was used to exclude doublets and analyze data.
Cell Surface Staining for TRAIL and DR5
Cells were harvested using enzyme-free cell dissociation buffer (Life Technologies). Cells were washed with FACS buffer (PBS with 1% FBS and 0.1% sodium azide) and stained with conjugated antibodies against TRAIL and DR5 (BioLegend). Flow cytometry data was collected using an LSR II flow cytometer (BD Biosciences). FlowJo™ software was used to exclude doublets and analyze data.
Western blotting
Cells were harvested and lysed using RIPA cell lysis buffer (Sigma-Aldrich) with 1x protease inhibitor (Roche) and 1x phosphatase inhibitor (Roche). Protein was quantified using the Pierce BCA Protein Assay Kit (Life Technologies). Samples were prepared for gel loading in NuPAGE LDS sample buffer with 1x reducing agent (Thermo Fisher Scientific). Samples were loaded into 4–12% NuPAGE SDS-polyacrylamide gels (Invitrogen). Proteins were transferred to polyvinylidene difluoride membranes, and the membranes were blocked with 5% nonfat milk in TBST. Membranes were incubated overnight with primary antibodies, then with horseradish peroxidase labelled secondary antibodies. Chemiluminescent signal was detected using a Syngene imaging system. NIH ImageJ software was used to quantify intensity of bands.
Xenograft studies
All xenograft studies were conducted in accordance with the rules and regulations of the Fox Chase Cancer Center Institutional Animal Care and Use Committee (IACUC). Pathogen free conditions were confirmed, including absence of mouse hepatitis virus or Corynebacterium bovis infections. The mammary fat pads of 6–8 week old female athymic nude mice from Taconic [NCrFoxn1nu, genotype sp/sp] were inoculated with MDA-MB-231 or MDA-MB-468 breast cancer cells. Cells were suspended in PBS and injected into mice as a 1:1 suspension with Matrigel (BD Biosciences). Tumors established and reach a volume of 150–250 mm3 before mice were randomized and treatment with a vehicle control or ONC201 was initiated. ONC201 was given orally, as a 200 μL suspension containing 20% Kolliphor EL (Sigma-Aldrich), 10% DMSO, and 70% PBS. Mice were treated 1 or 3 times weekly and had tumor volumes and weights measured two times weekly.
Statistical analysis
To assess the statistical significance of differences, an unpaired Student’s t test was performed using the GraphPad t-test calculator (https://www.graphpad.com/quickcalcs/ttest1/). Bar graphs were annotated using the following guidelines: ns: p≥0.05; *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001. Comparisons were made against the vehicle treated control.
Results
ONC201 is efficacious against triple negative and non-triple negative breast cancer cells
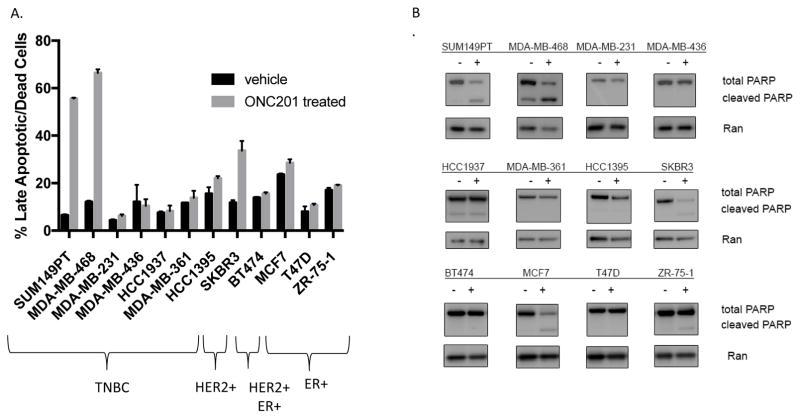
A panel of 13 TNBC (representing both basal-like and mesenchymal-like subtypes) and non-TNBC cell lines were treated with ONC201 and TRAIL. GI50 values were calculated from the resulting dose response curves (Table 1, Fig. S1). The results showed that irrespective of sensitivity to TRAIL, most breast cancer cell lines (11/13) had GI50 values for ONC201 in the low micromolar range. These doses are clinically achievable based on the results of pharmacokinetic studies conducted as part of the first-in-human trial of the compound [11]. Annexin V-PI staining was performed to quantify the apoptosis induced by the compound (Fig. 1A). Western blot analysis was used to examine PARP cleavage following treatment with the compound (Fig. 1B). A subset of TNBC (2/8) and non-TNBC (2/5) underwent apoptotic cell death. Cell lines which showed high levels of apoptosis in the annexin V-PI staining assay also showed a decrease in total PARP and an increase in cleaved PARP in the western blots. The two TNBC cell lines that underwent apoptosis were the most sensitive to the pro-apoptotic effects of the compound, with 55–70% of the cells becoming both annexin V/PI positive following treatment with 10 μM of ONC201 (Fig. 1A). The non-TNBC cell line that underwent apoptosis did so to a lesser extent, with no more than 40% of the cells becoming annexin V/PI positive following ONC201 treatment (Fig. 1A). Overall these results show that ONC201 induces cell death in both TNBC and non-TNBC cells, and that the effect is more potent in TNBC cells.
Table 1. ONC201 shows efficacy in triple negative breast cancer cells regardless of sensitivity to TRAIL.
Breast cancer cells were treated with ONC201 for 72 hours or recombinant human TRAIL for 4 hours and resulting dose response curves were used to calculate GI50 values.
| Cell Line | ONC201 GI50 (μM) | TRAIL GI50 (ng/mL) | Subtype |
|---|---|---|---|
| MDA-MB-436 | ~100 | >1000 | TNBC |
| HCC1937 | ~100 | >1000 | TNBC |
| BT20 | 2 | >1000 | TNBC |
| HCC1395 | 5 | >1000 | TNBC |
| MDA-MB-361 | 5 | >1000 | TNBC |
| MDA-MB-468 | 2 | 250 | TNBC |
| SUM149PT | 2 | 125 | TNBC |
| MDA-MB-231 | 5 | 250 | TNBC |
| SKBR3 | 4 | >1000 | HER2 amplified |
| BT474 | 5 | >1000 | ER+, HER2 amplified |
| T47D | 6.25 | >1000 | ER+ |
| MCF7 | 12.5 | >1000 | ER+ |
| ZR-75-1 | 6.25 | >1000 | ER+ |
Figure 1. ONC201 induces cell death in TNBC and non-TNBC cells.
A) Annexin-V/PI double positive cells were quantified using flow cytometry following a 72 hour treatment with a vehicle control or 10 μM ONC201 (n=2 experiments for each cell line). B) Western blot of TNBC cells treated with a vehicle control or 10 μM ONC201 for 72 hours to compare PARP cleavage. ns: p≥0.05; *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001.
Cell death in TNBC cells activates the extrinsic apoptosis pathway, is TRAIL dependent, and translates to an anti-tumor effect in vivo
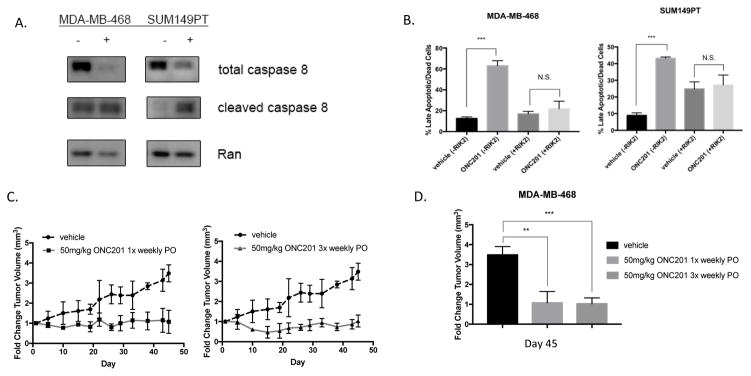
TNBC cells that underwent cell death following treatment with ONC201 were also sensitive to TRAIL (Table 1, Fig. 1). These cells showed a decrease in total caspase-8 and an increase in cleaved caspase-8 (Fig. 2A), while those that did not undergo cell death do not (Fig. S2C). Given the previously reported ability of ONC201 to induce cell death through induction of TRAIL and DR5 [3], we hypothesized that its pro-apoptotic effects in TNBC were TRAIL-dependent. SUM149PT cells showed increased expression of surface TRAIL and increased levels of mature DR5 protein following treatment with ONC201 (Fig. S2A, B). Apoptosis in SUM149PT and MDA-MB-468 cells was blocked by co-incubation with a TRAIL-neutralizing antibody, RIK2 (Fig. 2B). Importantly, ONC201 showed single agent efficacy in the MDA-MB-468 xenograft model when given orally at a dose of 50 mg/kg once and three times weekly. Growth of xenograft tumors was prevented by treatment with the compound (Fig. 2C) and the differences in the change in tumor volume at day 45 between mice treated with a vehicle control or ONC201 were significant (Fig. 2D). No difference in efficacy was observed between a once weekly versus three times weekly dosing schedule (Fig. 2D). In addition, ONC201 showed effects that were possibly additive in combination with FDA approved drugs in MDA-MB-468 TNBC cells (Fig. S3A, S3B). These results indicate that the induction of cell death by ONC201 in TNBC cells involves activation of the extrinsic apoptosis pathway, is dependent on TRAIL, and leads to in vivo anti-tumor efficacy of the compound.
Figure 2. The pro-apoptotic effects of ONC201 in some TNBC cells involve the extrinsic pathway, are TRAIL-dependent, and translate to efficacy in the MDA-MB-468 breast cancer xenograft model.
A) Western blot of MDA-MB-468 and SUM149PT TNBC cells treated with a vehicle control or 10 μM ONC201 for 72 hours to show caspase-8 cleavage. B) Annexin-V/PI double positive cells were quantified using flow cytometry following a 72 hour treatment with a vehicle control or ONC201 (MDA-MB-468: 5 μM, SUM149PT: 10 μM), with or without 1 μg/mL RIK2 TRAIL blocking antibody (n=2 experiments for each cell line). C) Fold change tumor volume measured over time in nude mice bearing MDA-MB-468 xenografted tumors treated orally with a vehicle control (n=3), 50 mg/kg ONC201 once per week (n=3), or 50 mg/kg ONC201 three times per week (n=4). D) Comparison of fold change tumor volume in mice treated with a vehicle control, 50 mg/kg ONC201 once per week, or 50mg/kg ONC201 three times per week on day 45 of the experiment. ns: p≥0.05; *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001.
TNBC cells which do not undergo apoptosis show differential sensitivity to the anti-proliferative effects of ONC201
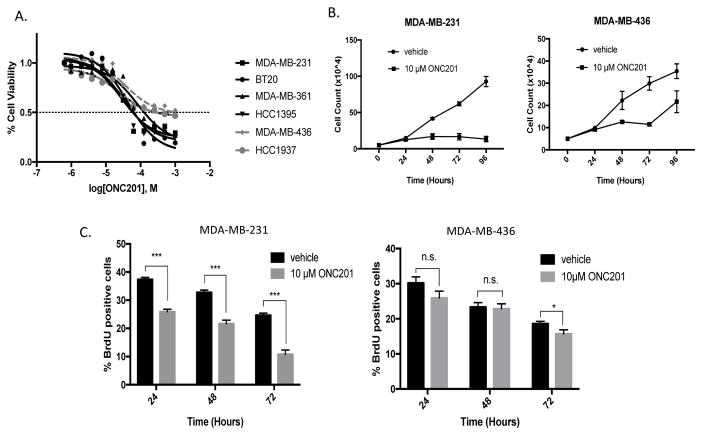
ONC201 treatment decreased the number of viable cells in the TNBC cells that did not undergo apoptosis (Fig. 3A). In 2 of 6 cell lines tested showed less sensitivity to the compound, characterized by a failure of the cell viability to drop below 50%, even at a dose of 100 μM of ONC201 (Fig. 3A). The observation that ONC201 decreased the number of viable TNBC cells without inducing apoptosis suggested that the compound exerted an anti-proliferative effect. Count of viable cells over time showed that cell proliferation was inhibited over time in sensitive MDA-MB-231, MDA-MB-361, and HCC1395 cells (Fig. 3B, Fig. S4A) more than in relatively resistant MDA-MB-436 cells (Fig. 3B). Uptake of nucleoside analog BrdU was decreased by greater than 10% at 24, 48, and 72 hours of ONC201 treatment in sensitive MDA-MB-231 cells, but not in relatively resistant MDA-MB-436 cells (Fig. 3C).
Figure 3. Differential sensitives to anti-proliferative effects of ONC201 are observed among TNBC cells that do not undergo apoptosis.
A) Dose response curves of TNBC cells treated with ONC201 for 72 hours. B) Trypan blue exclusion used to determine viable cell count in TNBC cells over time following treatment with a vehicle control or 10 μM ONC201. C) Percent BrdU-positive cells over time in TNBC cells following treatment with a vehicle control or 10 μM ONC201. ns: p≥0.05; *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001.
In TNBC cells with high sensitivity, the compound activates an integrated stress response and causes cells to accumulate in G1
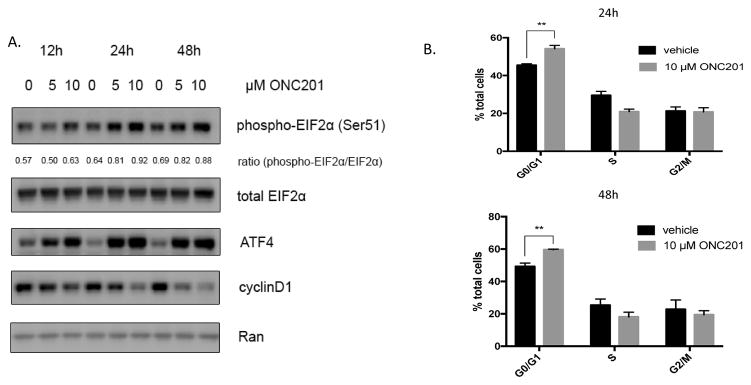
Treatment of MDA-MB-231 cells with 5 μM or 10 μM of ONC201 led to a dose-dependent activation of the ISR characterized by increased phosphorylation of the eukaryotic initiation factor 2α (eIF2α) at serine 51, induction of ATF4, and a decrease in cyclin D1 protein levels as early as 12 hours (Fig. 4A). This was associated with an accumulation of cells in the G1 phase of the cell cycle, shown by cell cycle profiling of unsynchronized cells (Fig. 4B). These results show that ONC201 has an anti-proliferative effect in cells that do not undergo cell death, and those with high sensitivity activate the ISR and accumulate in G1.
Figure 4. ONC201 activates an integrated stress response, decreases cyclin D1 expression, and causes an accumulation of cells in G1 in TNBC cells sensitive to the compounds anti-proliferative effects.
A) Western blot analysis of MDA-MB-231 cells for EIF2α phosphorylation, ATF4 expression, and cyclin D1 expression and following treatment with a vehicle control, 5 μM, or 10 μM ONC201 for 72 hours. Densitometry was performed using NIH ImageJ software. The ratio represents the intensity of the phospho-EIF2α band divided by the intensity of the total EIF2α band. B) Quantification of cells in G0/G1, S, and G2 phases of the cell cycle using flow cytometric analysis of propidium iodide stained MDA-MB-231 cells at 24 and 48 hours following treatment with a vehicle control or 10 μM ONC201. ns: p≥0.05; *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001.
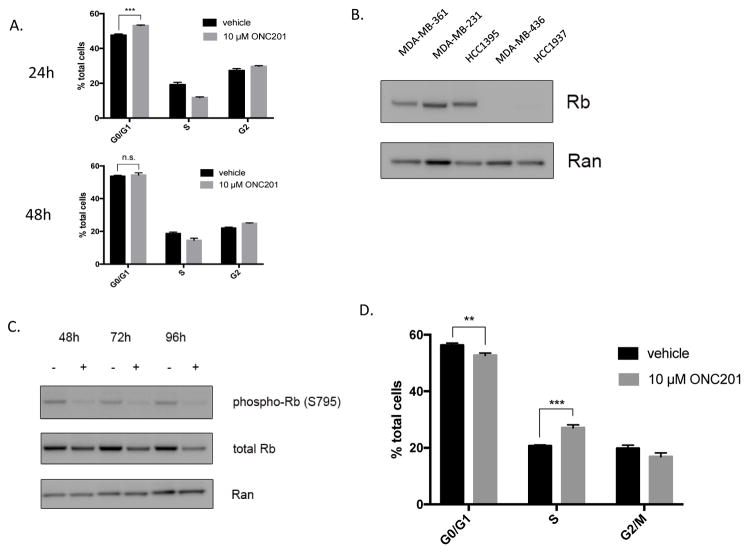
pRb levels may be important for ONC201 to arrest and maintain cells in the G1 phase of the cell cycle
Relatively resistant MDA-MB-436 cells did not arrest in G1 following treatment with ONC201 (Fig. 5A). Expression of pRb was associated with increased sensitivity to the anti-proliferative effects of ONC201, and relatively resistant MDA-MB-436 and HCC1937 did not express detectable levels of the pRb protein (Fig. 5B). ONC201 treatment decreased total pRb protein in MDA-MB-231 cells over time (Fig. 5C). By 72 hours of ONC201 treatment, MDA-MB-231 cells passed through the restriction point from G1 into S phase (Fig. 5D), but did not proliferate (Fig. 3B, 3C). Treatment with 100 mg/kg of ONC201 orally once per week as a single agent did not lead to an anti-tumor effect in the MDA-MB-231 xenograft model (Fig. S4B, S4C). These data show that ONC201 has an anti-proliferative effect in TNBC cells that do not undergo apoptosis and that pRb expression may be important for initiation and maintenance of G1 arrest.
Figure 5. Rb levels during ONC201 induced growth arrest in the G1 phase of the cell cycle.
A) Quantification of cells in G0/G1, S, and G2 phases of the cell cycle using flow cytometric analysis of propidium iodide stained MDA-MB-436 cells at 24 and 48 hours following treatment with a vehicle control or 10 μM ONC201. B) Western blot analysis of baseline pRb expression in TNBC cells. C) Western blot analysis of MDA-MB-231 cells treated with a vehicle control or 10 μM ONC201 over time probed to examine changes in levels of phosphorylated and total Rb. D) Quantification of cells in G0/G1, S, and G2 phases of the cell cycle using flow cytometric analysis of propidium iodide stained MDA-MB-436 cells at 72 hours following treatment with a vehicle control or 10 μM ONC201.ns: p≥0.05; *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001.
ONC201 synergizes with taxanes in tumor cells with decreased sensitivity to ONC201 as a single agent
We further explored the potential for synergies between ONC201 and FDA-approved anti-cancer drugs. Combinations with FDA approved therapies that synergized with ONC201 in TNBC cells with relative resistance to the anti-proliferative effects of the compound were identified. Combination indices (CIs) calculated using the Chou-Talalay method [17] from cell viabilities following drug treatment showed that ONC201 synergized with taxanes docetaxel and paclitaxel in MDA-MB-436 and HCC1937 (BRCA1-deficient) TNBC cell lines (Table 2). These results demonstrate that the combination of ONC201 and taxanes, which are used as first-line treatment of breast cancer in both the adjuvant and metastatic settings, is synergistic.
Table 2. ONC201 synergizes with docetaxel and paclitaxel in TNBC cells with decreased sensitivity to ONC201.
Combination indices for MDA-MB-436 and HCC1937 cells treated with different concentrations of ONC201 and paclitaxel for 72 hours. Combination indices calculated using the Chou-Talalay method. CI>1: antagonism, CI=1: additive effect, CI<1: synergy.
| MDA-MB-436 | HCC1937 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CI | μM ONC201 | CI | μM ONC201 | ||||||
| nM paclitaxel | 2.5 | 5 | 10 | 20 | nM paclitaxel | 2.5 | 5 | 10 | 20 |
| 6.25 | 0.38 | 0.56 | 0.54 | 0.86 | 6.25 | 0.55 | 0.44 | 0.67 | 1.18 |
| 12.5 | 0.71 | 0.52 | 0.43 | 0.68 | 12.5 | 0.53 | 0.51 | 0.7 | 1.16 |
| 25 | 0.59 | 0.42 | 0.38 | 0.41 | 25 | 0.67 | 0.68 | 0.9 | 1.32 |
| 50 | 0.98 | 0.66 | 0.65 | 0.68 | 50 | 1.17 | 1.05 | 1.24 | 1.69 |
| CI | μM ONC201 | CI | μM ONC201 | ||||||
| nM docetaxel | 2.5 | 5 | 10 | 20 | nM docetaxel | 2.5 | 5 | 10 | 20 |
| 6.25 | 0.73 | 0.39 | 0.41 | 0.63 | 6.25 | 0.45 | 0.34 | 0.44 | 0.75 |
| 12.5 | 0.58 | 0.44 | 0.38 | 0.55 | 12.5 | 0.67 | 0.45 | 0.55 | 0.78 |
| 25 | 0.46 | 0.45 | 0.46 | 0.54 | 25 | 0.84 | 0.68 | 0.78 | 1 |
| 50 | 0.39 | 0.79 | 0.78 | 0.83 | 50 | 1.62 | 1.25 | 1.33 | 1.49 |
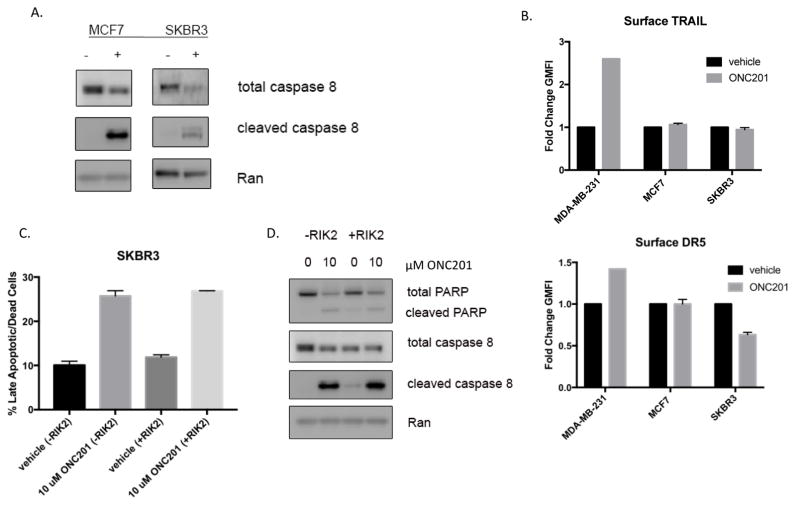
ONC201 activates caspase-8 cleavage and induces TRAIL-independent cell death in non-TNBC cells
We initially hypothesized that because ONC201 has effects on mechanisms of TRAIL resistance, the compound could show efficacy in non-TNBC cells. Unlike TRAIL, ONC201 induced cell death in non-TNBC cells (Fig. 1A, 1B). At 72 hours, treatment with 10 μM ONC201 decreased expression of anti-apoptotic proteins from the Bcl-2 and IAP families, regardless of whether the cells underwent cell death (Fig. S5). Non-TNBC cells SKBR3 and MCF7 showed a decrease in total caspase-8 and an increase in cleaved caspase-8 following treatment with 10 μM ONC201 for 72 hours (Fig. 6A). While ONC201 induced expression of surface TRAIL and DR5 in control MDA-MB-231 cells, it did not induce these components of the extrinsic apoptosis pathway in SKBR3 and MCF7 cells (Fig. 6B). Unlike the apoptosis induced by ONC201 in TNBC cells, apoptosis in non-TNBC cells was not abrogated by TRAIL neutralizing antibody RIK2 (Fig. 6C, 6D). This data indicates that ONC201 may induce cell death in non-TNBC cells through a TRAIL-independent mechanism.
Figure 6. The pro-apoptotic effects of ONC201 in non-TNBC cells involve caspase 8 cleavage, but are TRAIL-independent.
A) Immunoblot analysis of SKBR3 and MCF7 cells treated with a vehicle control or 10μM ONC201 for 72 hours. B) Geometric mean fluorescence intensity of TRAIL and DR5 surface staining was determined in MDA-MB-231 and MCF7 cells treated with a vehicle control or 5 or 10μM ONC201 for 72 hours. C) Annexin-V/PI double positive cells were quantified in SKBR3 using flow cytometry following a 72 hour treatment with a vehicle control or 10μM ONC201 in the presence or absence of 1 ug/mL RIK2 TRAIL blocking antibody. D) Immunoblot of MCF7 cells treated with a vehicle control or 10μM ONC201 in the presence or absence of 1 ug/mL RIK2 TRAIL blocking antibody.
Discussion
Here, we demonstrate that ONC201 shows efficacy in a wide range of breast cancer subtypes, independent of their sensitivity to TRAIL. TRAIL has been of interest as a cancer therapeutic due to its ability to induce cell death in cancer cells while sparing normal cells [2], but many breast cancers show resistance [8]. ONC201 was initially identified as a small molecule inducer of the TRAIL pathway [3] and cell death. In breast cancer cells, we show that ONC201 has anti-proliferative and TRAIL-independent effects as well. This is highly relevant to breast cancer, where most subtypes are resistant to TRAIL, and explains the observed broad efficacy profile of ONC201. Most TNBC (n=6) and all non-TNBC (n=5) cells show GI50 values in the low micromolar range following treatment with ONC201. This is the first demonstration of ONC201 efficacy in non-TNBC cells, and is clearly different than what was observed with TRAIL, where almost all (n=10) breast cancer cells show resistance.
ONC201 was previously shown to induce cell death through activation of the extrinsic apoptosis pathway. We performed annexin V-PI staining and western blotting for PARP cleavage to determine if ONC201 induced cell death in breast cancers. Cell death is induced in a subset of TNBC (2/8) and non-TNBC (4/5) cells. In TNBC cells where ONC201 induces cell death, caspase-8 cleavage indicates that the extrinsic pathway is being activated. Use of a TRAIL-neutralizing antibody abrogates apoptosis and confirms that the cell death is TRAIL-dependent. These observations agree with the mechanisms of action of the compound that have been previously established by our lab [3,6]. The pro-apoptotic effects of ONC201 in TNBC translate to an anti-tumor effect in the in vivo MDA-MB-468 xenograft model. In addition, ONC201 has effects that are potentially additive in combination with FDA approved drugs in MDA-MB-468 cells.
ONC201 acts through TRAIL-independent mechanisms in breast cancer as well. In TNBC cells where ONC201 does not induce cell death, the compound has an anti-proliferative effect and activates an ISR, decreases expression of cyclin D1 and leads to accumulation of cells in the G1 phase of the cell cycle. Reduced expression of cyclin D1 may account for the growth arrest of breast tumors cells in G1 after ONC201 exposure, and may be a useful biomarker in post-treatment biopsies in the clinic. The ISR is a cellular response that regulates protein synthesis. Various stresses can trigger phosphorylation of eukaryotic initiation factor 2α (eIF2α), leading to a generalized inhibition of protein translation combined with specific expression of stress-response genes such as ATF4 (reviewed by Quirós et al.) [18]. Activation of the ISR and phosphorylation of eIF2α by kinases PKR and PERK induces proteasome-dependent degradation of cyclin D1 [19]. Our lab previously showed that the ISR activated by ONC201 involves eIF2α kinases HRI and PKR [6]. This suggests that the anti-proliferative effects of ONC201, in addition to its pro-apoptotic effects, may be mediated through the ISR. Future studies will need to elucidate the exact role of the ISR in the anti-proliferative effects of ONC201.
Not all TNBC cells are sensitive to the anti-proliferative effects of ONC201. In 2 of 6 TNBC cell lines we observed increased resistance to ONC201, which was associated with decreased expression of pRb at the protein level. pRb is an important tumor suppressor that regulates passage through the G1/S restriction point of the cell cycle by inhibiting E2F transcription factors [20]. Phosphorylation of pRb inactivates the protein and relieves inhibition of the E2F transcription factors, allowing passage through the restriction point and into S phase. In the presence of growth promoting signals, cells lacking pRb will pass through the G1/S restriction point in an unrestricted manner, regardless of expression of upstream cell cycle regulators such as cyclin D1. Further evidence that pRb may be important for ONC201-mediated G1 arrest is provided by the observation that ONC201 decreases expression of total pRb in MDA-MB-231 cells, and by 72 hours the cells that previously had accumulated in G1 move into S phase. Although the cells have entered S phase, both cell counting and BrdU uptake assays show that they are not actively proliferating. The anti-proliferative effect of ONC201 is not sufficient to translate to an anti-tumor effect in vivo, and MDA-MB-231 xenograft tumors do not respond to treatment with 100 mg/kg ONC201 orally once per week. These results prompted a search for a combination with an approved drug in TNBC to potentiate the anti-tumor effect of ONC201. In TNBC cells with decreased sensitivity to ONC201 as a single agent, the compound synergizes with taxanes paclitaxel and docetaxel. Taxanes are used as first-line chemotherapy agents to treat TNBC in the adjuvant as well as the metastatic disease setting. High levels of anti-apoptotic proteins are associated with resistance to chemotherapeutic drugs (reviewed by Pommier et al [21]). The ability of ONC201 to decrease expression of anti-apoptotic proteins [6,9] may contribute to the observed synergy between the compound and taxane chemotherapy. While the combination of ONC201 and taxanes in breast cancer needs to be tested in vivo and further understood mechanistically, there is potential for translation to the clinic.
SKBR3 and MCF7 non-TNBC cells show induction of caspase-8 and PARP cleavage following treatment with ONC201. This observation is significant given the high levels of TRAIL resistance in these cells. We initially hypothesized that ONC201 could be sensitizing cells to the apoptotic effects of TRAIL through previously characterized effects on mechanisms of TRAIL-resistance. ONC201 treatment decreases levels of IAP and Bcl-2 family proteins in non-TNBC cells, high expression of which has been shown to be a regulator of sensitivity to TRAIL-induced apoptosis [16]. ONC201 treatment has also been shown to upregulate cell surface DR5 [3], low levels of which are associated with resistance to TRAIL [15]. Surprisingly, treatment with ONC201 did not upregulate surface TRAIL and DR5 in SKBR3 and MCF7 cells as it did in control MDA-MB-231 cells, and TRAIL-neutralizing antibody RIK2 failed to abrogate cell death induced by the compound in the cells. These observations suggest that ONC201 is acting through a TRAIL-independent mechanism of action. Fas ligand (FasL) and tumor necrosis factor α (TNFα) can activate death receptors and thus apoptosis through the extrinsic pathway (reviewed by Tait et al.) [22]. It is possible that these alternative death receptor ligands are responsible for the effects of ONC201 in these cells or that feedback mechanisms from downstream caspase activation result in caspase-8 cleavage and amplification of a cell death signal independent of involvement of the death receptors. Further experiments are needed to explore this.
In summary, this preclinical study demonstrates that ONC201 shows efficacy in TNBC and non-TNBC cells including BRCA1-deficient cells, regardless of their sensitivity to TRAIL. Some TNBC cells undergo TRAIL-dependent cell death that translates to an anti-tumor effect in vivo, but the effects of ONC201 in most TNBC cells are anti-proliferative rather than pro-apoptotic (2/8). Expression of pRb may be important for sensitivity to the anti-proliferative effects of the compound. This is the first demonstration of the efficacy of ONC201 across a spectrum of breast cancer cell lines including non-TNBC cells that are TRAIL-resistant. ONC201 induces cell death that appears to be TRAIL-independent in non-TNBC cells. The heterogeneity of breast cancers observed at the genomic and epigenomic level may explain why ONC201 exerts such a wide range of effects among cells of this tumor type. Further studies are needed to elucidate the exact mechanisms responsible for the TRAIL-independent effects of ONC201. ONC201 has completed its first-in-human trial in advanced solid tumors, where it was shown to be safe and to have preliminary efficacy [11]. It is currently being tested in multiple phase II trials of patients with solid tumors and hematological malignancies. Overall our findings present a preclinical rationale for clinical testing of ONC201 as a single agent and in combination with approved therapies, including taxanes, in breast cancers.
Supplementary Material
Acknowledgments
Financial Support: The authors acknowledge NIH grant R01 CA173453 (W.S.E-D.) and support from an American Cancer Society Research Professorship (W.S.E-D.).
Footnotes
Note: This work was presented as part of the AACR-NCI-EORTC International Conference on Molecular Targets and Therapeutics (November 2015), and in an updated version as part of the 107th Annual Meeting of the American Association for Cancer Research (April 2016).
References
- 1.Allen JE, et al. Discovery and clinical introduction of first-in-class imipridone ONC201. Oncotarget. 2016 doi: 10.18632/oncotarget.11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JE, et al. Identification of TRAIL-inducing compounds highlights small molecule ONC201/TIC10 as a unique anti-cancer agent that activates the TRAIL pathway. Mol Cancer. 2015 doi: 10.1186/s12943-015-0346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen JE, et al. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med. 2013 doi: 10.1126/scitranslmed.3004828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walczak H, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 5.Rahman M, et al. TRAIL induces apoptosis in triple-negative breast cancer cells with a mesenchymal phenotype. Breast Cancer Res Treat. 2009;113:217–230. doi: 10.1007/s10549-008-9924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kline CLB, et al. ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2α kinases. Sci Signal. 2016;9:ra18. doi: 10.1126/scisignal.aac4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prabhu VV, Allen JE, Dicker DT, El-Deiry WS. Small-Molecule ONC201/TIC10 Targets Chemotherapy-Resistant Colorectal Cancer Stem-like Cells in an Akt/Foxo3a/TRAIL-Dependent Manner. Cancer Res. 2015 doi: 10.1158/0008-5472.CAN-13-3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen JE, Crowder R, El-Deiry WS. First-In-Class Small Molecule ONC201 Induces DR5 and Cell Death in Tumor but Not Normal Cells to Provide a Wide Therapeutic Index as an Anti-Cancer Agent. PLoS One. 2015;10:e0143082. doi: 10.1371/journal.pone.0143082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karpel-Massler G, et al. TIC10/ONC201 synergizes with Bcl-2/Bcl-xL inhibition in glioblastoma by suppression of Mcl-1 and its binding partners in vitro and in vivo. Oncotarget. 2015;6 doi: 10.18632/oncotarget.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishizawa J, et al. ATF4 induction via an atypical integrated stress response by small molecule ONC201 induces p53-independent apoptosis in hematological malignancies. doi: 10.1126/scisignal.aac4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein MN, Bertino J, Kaufman HL, Mayer TM, Moss RA, Silk AW, Chan N, Rodriguez L, Hafferty BG, DiPaola RS, Saunders T, Beckett Y, Zheng L, El-Deiry WS, Allen JE, Stogniew M, Oster W, Mehnert JM. First-in-human clinical trial of ONC201 in patients with refractory sold tumors. Clin Cancer Res. 2017 doi: 10.1158/1078-0432.CCR-16-2658. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neve RM, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehmann BDB, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahman M, Pumphrey J, Lipkowitz S. The TRAIL to targeted therapy of breast cancer. Adv Cancer Res. 2009;103:43–73. doi: 10.1016/S0065-230X(09)03003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhang B. TRAIL resistance of breast cancer cells is associated with constitutive endocytosis of death receptors 4 and 5. Mol Cancer Res. 2008;6:1861–71. doi: 10.1158/1541-7786.MCR-08-0313. [DOI] [PubMed] [Google Scholar]
- 16.Garimella SV, et al. Identification of novel molecular regulators of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in breast cancer cells by RNAi screening. Breast Cancer Res. 2014;16:R41. doi: 10.1186/bcr3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou TC. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 18.Quirós PM, Mottis A, Auwerx J. Mitonuclear communication in homeostasis and stress. Nat Rev Mol Cell Biol. 2016;17:213–226. doi: 10.1038/nrm.2016.23. [DOI] [PubMed] [Google Scholar]
- 19.Raven JF, et al. PKR and PKR-like endoplasmic reticulum kinase induce the proteasome-dependent degradation of cyclin D1 via a mechanism requiring eukaryotic initiation factor 2?? phosphorylation. J Biol Chem. 2008;283:3097–3108. doi: 10.1074/jbc.M709677200. [DOI] [PubMed] [Google Scholar]
- 20.Nevins JR. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science (80-) 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 21.Pommier Y, Sordet O, Antony S, Hayward RL, Kohn KW. Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene. 2004;23:2934–49. doi: 10.1038/sj.onc.1207515. [DOI] [PubMed] [Google Scholar]
- 22.Tait SWG, Green D. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010 doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.