Abstract
The purpose of this study was to identify markers from perfusion, diffusion, and chemical shift imaging in glioblastomas (GBMs) and to correlate them with genetically determined and previously published patterns of structural magnetic resonance (MR) imaging. Twenty-six patients (mean age 60 years, 13 female) with GBM were investigated. Imaging consisted of native and contrast-enhanced 3D data, perfusion, diffusion, and spectroscopic imaging. In the presence of minor necrosis, cerebral blood volume (CBV) was higher (median ± SD, 2.23% ± 0.93) than in pronounced necrosis (1.02% ± 0.71), pcorr = 0.0003. CBV adjacent to peritumoral fluid-attenuated inversion recovery (FLAIR) hyperintensity was lower in edema (1.72% ± 0.31) than in infiltration (1.91% ± 0.35), pcorr = 0.039. Axial diffusivity adjacent to peritumoral FLAIR hyperintensity was lower in severe mass effect (1.08*10–3 mm2/s ± 0.08) than in mild mass effect (1.14*10–3 mm2/s ± 0.06), pcorr = 0.048. Myo-inositol was positively correlated with a marker for mitosis (Ki-67) in contrast-enhancing tumor, r = 0.5, pcorr = 0.0002. Changed CBV and axial diffusivity, even outside FLAIR hyperintensity, in adjacent normal-appearing matter can be discussed as to be related to angiogenesis pathways and to activated proliferation genes. The correlation between myo-inositol and Ki-67 might be attributed to its binding to cell surface receptors regulating tumorous proliferation of astrocytic cells.
Keywords: Radiogenomics, glioblastoma, diffusion imaging, perfusion imaging, proton spectroscopy
Introduction
Radiogenomics is a field of research that analyses correlations between gene expression and magnetic resonance imaging (MRI) patterns. For glioblastoma multiforme (GBM) this has been described by several authors.1–4 Diehn et al. searched for an association between 10 binary imaging traits and gene expression data from the corresponding tissue probes in glioblastoma, the imaging features being: edematous or infiltrative pattern of T2-hyperintensity, degree of contrast enhancement, presence or absence of necrosis, cortical involvement, subventricular zone involvement, mass effect, assessment of the proportion of contrast-enhancing tumor to necrosis, and of contrast-enhancing tumor to T2-fluid-attenuated inversion recovery (FLAIR) hyperintensity, degree of edema, and T2 heterogeneity. The most prominent radiogenomic findings in this work were (i) correlation of the contrast-enhancement/necrosis ratio with epidermal growth factor receptor (EGFR) overexpression, (ii) association of the infiltrative radiophenotype with predominantly proneural marker genes, and (iii) mass effect with proliferation genes.2
This means that in each GBM an assignment to these MR imaging patterns mirrors the genetics of the tumor. Based on The Cancer Genome Atlas (TCGA) network with its included gene information and MRI,5,6 several studies investigated the connection between certain genetic characteristics of GBM and associated imaging traits.7–11 They focused on structural MR imaging without using perfusion, diffusion or spectroscopic imaging. In contrast, Jain et al.12,13 and Gupta et al.14 included perfusion-weighted imaging into the radiogenomic analysis and could show an influence of EGFR gene mutation or amplification on the cerebral blood volume (CBV) in GBM. Recently, a set of 19 recurrent glioblastomas investigated by dynamic contrast enhanced (DCE) perfusion and diffusion tensor imaging (DTI) has been added to the TCIA database.15
Perfusion and diffusion-weighted imaging can be subsumed under the term “mesoscopic” imaging, because they are aimed at bridging the gap between the tissue microstructure on a micrometer scale and the macroscopic resolution of MRI, which is above a millimeter. Vessel size (VS) imaging may serve as an example for evaluating the mean capillary diameter (about 7 µm in a normal brain) by using MRI with the resolution of about 2 mm.16–19 Diffusion-weighted imaging being sensitive to the structural tissue organization on a sub-voxel scale is another good example of mesoscopic imaging.20 Moreover, metabolites derived by chemical shift imaging (CSI) mirror the intra- and intercellular metabolism, e.g. N-acetylaspartate (NAA) and N-acetylaspartylglutamate for the communication of neurons, oligodendrocytes and astrocytes.21
Motivated by the results regarding CBV, the purpose of this retrospective case study was to find out whether perfusion-derived parameters such as CBV and VS, diffusion metrics (mean diffusivity, MD; axial diffusivity, AD; radial diffusivity, RD; fractional anisotropy, FA) and metabolites from CSI (NAA; creatine, Cr; choline-containing compounds, Cho; myo-inositol, mI; sum of glutamine and glutamate, Glx) are also related to radiogenetic characteristics of GBMs. The joint use of these MRI techniques in one radiogenomic patient cohort has not been reported before. Also, the contribution of VS imaging to radiogenomic correlations has not yet been assessed. We hope to provide new insights in these areas.
The three most prominent radiogenomic imaging traits of Diehn et al.2 were used as surrogate markers for gene expression for what is hereafter addressed as “radiophenomic” classification. The chosen traits were therefore (i) presence of minor or pronounced necrosis, (ii) edematous or infiltrative pattern of T2-hyperintensity, and (iii) mass effect.
Methods
Study population
Twenty-six treatment-naïve primary GBM patients (mean age 60.6 years, range 32–82 years), 13 females, were retrospectively included in this case study. The study was approved by the ethical committee of the University of Freiburg (approval number 565/15) and performed in accordance with the ethical standards of the Declaration of Helsinki from 1964 and its amendments.
Patients with preoperative three-dimensional (3D) data sets for operation planning and fiber tracking of eloquent fiber bundles such as the pyramidal tract or optic radiation and two-dimensional (2D) chemical shift imaging (CSI, in 17/26 patients) were included in the study.
MRI and spectroscopy
MRI was performed on a 3T system (Magnetom TIM TRIO, Siemens, Erlangen, Germany) using a 12-channel head coil. The imaging protocol consisted of a 3D T2-weighted fluid-attenuated sequence (repetition time (TR), 5000 ms; effective echo time (TEeff), 388 ms; inversion time (TI), 1800 ms; flip angle, variable; pixel size, 1 mm3), a 3D T1-weighted magnetization prepared rapid gradient echo sequence (TR, 1390 ms; TE, 2.15 ms; TI, 800 ms; flip angle,15 degrees; pixel size, 1 mm3) was acquired before and after perfusion imaging with application of 17 ml 0.5 M gadobenate dimeglumine (Multihance®, Bracco, Konstanz, Germany), followed by a chaser of 60 ml NaCl 0.9% solution for perfusion imaging, flow rate 3 ml/s. Perfusion imaging consisted of 2D serial, single-shot, double-echo readout echo planar imaging (EPI) sequences (40 dynamic scans, bandwidth 2273 Hz/px, TR, 2000 ms; TEGE, 21 ms; TESE, 94 ms; pixel size, 2.5 × 2.5 × 5 mm3, 6/8 partial Fourier) during bolus passage.22 For diffusion imaging, a diffusion-sensitive single-shot spin-echo EPI sequence with distortion correction was applied (61 diffusion encoding gradient directions; b-value, 0, 1000 s/mm2; TR, 8800 ms; TE, 102 ms; pixel size, 2 × 2 × 2 mm3).23
For 2D CSI, a point-resolved spectroscopy (PRESS) sequence with outer volume saturation was used (TR, 1500 ms; TE, 30 ms; voxel size, 10 × 10 × 15 mm3).
Routine follow-up MRI every three months consisted of T2- and dark fluid-weighted, native T1-weighted sequences, perfusion imaging (same amount of contrast medium), diffusion-weighted imaging with three directions and two b-values of 0 and 1000 s/mm2 for calculation of trace and apparent diffusion coefficient (ADC), and a 3D T1-weighted sequence after contrast application.
Post-processing, evaluation and clinical data
Perfusion data were processed by T1 leakage correction, estimation of the arterial input function (AIF), and calculation of the VS and the CBV as described by Kellner et al. and by the literature cited there.22 In brief, from the relaxation rates, the CBV and the VSI were calculated using the relations:
where and denote the relaxation rate time courses of the gradient and spin echo, respectively, and D denotes the ADC. CBV was normalized to a whole-brain median value of 3.2%, as in the works of Jain et al.12,13
Diffusion data were processed by using a MATLAB-based toolbox for fiber tracking.24 The effective self-diffusion tensor was computed on diffusion data corrected for motion and distortion artifacts. Maps of MD, AD, RD and FA were calculated using the relations between the sorted eigenvalues e1, e2, e3:
Spectra were evaluated by using LC Model.25 NAA and N-acetylaspartylglutamate were summarized as “NAA”, the choline-containing compounds glycerophosphocholine and phosphocholine as “Cho,” and glutamate and glutamine as “Glx.” Creatine (Cr) and myo-inositol (mI) were also fitted. In contrast to the imaging data, normalization to the contralateral normal appearing white matter was performed. To address those relative values, all metabolites will have an “n” for “normalized” as prefix such as nmI = mIipsi/mIcontra, nCho = Choipsi/Chocontra, nCr = Crispi/Crcontra, nGlx = Glxipsi/Glxcontra, and nNAA = NAAipsi/NAAcontra. One voxel from each of the following regions was chosen: contrast-enhancing tumor, non-enhancing tumor, normal-appearing matter (NAM) adjacent to the tumor edge and contralateral NAM. The latter was used for normalization to the contralateral side. In case of several available spectra, the one with the smallest full width at half maximum (FWHM) from LCModel output and best baseline was chosen. The mean FWHM from the LCModel output of all included spectra was 0.068 ppm (range 0.038–0.143 ppm). In all cases, tumor size was sufficient to exclude partial volume effects within the spectroscopy voxels. Only those metabolites with Cramer-Rao bounds of less than 20% were included and used for statistical evaluation.
Anatomical images, perfusion and diffusion maps were co-registered without reslicing for further evaluation and were available in an in-house 3D evaluation tool. A region of interest (ROI) analysis was performed. ROIs were drawn by consensus decision of T.D. and I.M. (two and 16 years of experience in neuroradiology, respectively). ROIs were: contrast-enhancing tumor (ce_tumour); whole area of T2/FLAIR hyperintense non-contrast-enhancing tumor without necrosis; T2/FLAIR hyperintense, non-contrast-enhancing tumor adjacent to the edge of the FLAIR hyperintense region (flair_edge); T2/FLAIR hyperintense, non-contrast-enhancing tumor adjacent to flair_edge (flair_hyper); NAM adjacent to but outside the FLAIR hyperintense region (NAM_outside), and contralateral NAM (NAM_contra). ROI placement is shown on a scheme in supplementary figure S1. Particular attention was paid to the edges of the ROIs, cerebrospinal fluid (CSF) space and to leptomeningeal vessels. To avoid partial volume effects, CSF and leptomeningeal vessels were excluded.
In case of recurrent tumor in the follow-up MR investigations, the 3D dark fluid-weighted sequences were co-registered in individual space by spm8, and the region of tumor recurrence was selected as an ROI in the original first data sets and maps (recurrence).
A classification according to the MRI traits described by Diehn et al.2 was performed by consensus reading of T.D. and I.M., and contained the following factors: presence of minor or pronounced necrosis, cortical involvement, subventricular zone involvement, mass effect, presence of edema or infiltration, qualitative assessment of the proportion of contrast-enhancing tumor to necrosis <1 or =1, qualitative assessment of the proportion of contrast-enhancing tumor/T2-FLAIR hyperintensity <1 or =1. For simplification and given the small number of cases, the following radiophenomic modules were finally included into statistical evaluation: (i) presence of minor or pronounced necrosis, (ii) none or mild versus moderate to severe mass effect, and (iii) presence of edema (with control of release of edema after surgery) or infiltration, Figure 1.
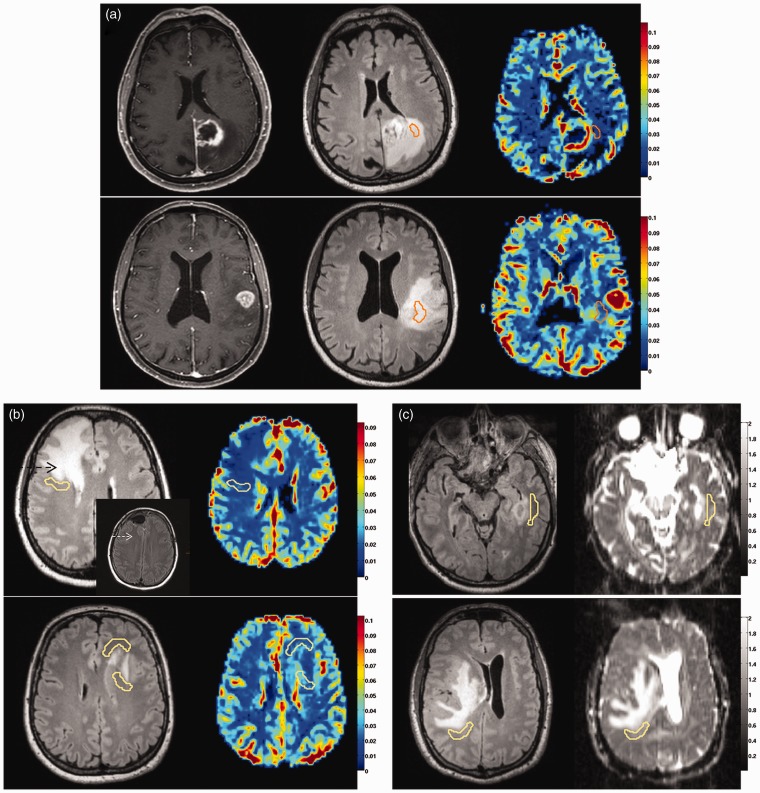
Figure 1.
Radiophenomic modules.
(a) Necrosis-related module with pronounced (upper row) or minor necrosis (lower row). From left to right: contrast-enhanced T1 image, FLAIR image, CBV map. The ROI shown is “flair_hyper”.
(b) Edema/infiltration-related module with edema (upper row, black arrow) and its release after operation (insert with white arrow), or presence of infiltration (lower row). The ROI “NAM_outside” is shown on the FLAIR images (left) and on the CBV maps (right).
(c) Proliferation-related module with mild or no mass effect (upper row) versus moderate to severe mass effect (lower row). The ROI “NAM_outside” is shown on the FLAIR images (left) and on the AD maps (right).
FLAIR: fluid-attenuated inversion recovery; CBV: cerebral blood volume; ROI: region of interest; NAM: normal-appearing matter.
Histopathological and immunostaining
Tissue samples were paraffin embedded after fixation in 4% phosphate-buffered formaldehyde and 4 µm thick sections were H&E stained according to standard protocols. Immunohistochemistry was performed according to standard protocols in an autostainer (Dako) after heat-induced epitope retrieval in citrate buffer. Primary antibodies used were Ki-67 (Mib1, 1:50, Dako) to determine the proliferation rate in the tumor and anti-IDH1-R123 (1:20, Dianova) to assess IDH1 mutations.
Statistics
All statistics were performed by SPSS 23.0.0.0 (IBM Corporation, Ehningen, Germany). Normal distribution was tested by Lilliefors-test. nCr, nCho, nNAA, and nGlx were normally distributed. Analysis according to the radiophenomics modules and to IDH1 mutation was performed by a two-sided t-test. RD, MD, AD, FA, VSI, CBV, and nmI were not normally distributed and analyzed by Mann-Whitney U test. A Chi square test was performed for the group minor/pronounced necrosis versus infiltration/edema. An analysis according to the recurrence versus normal-appearing tissue (NAM_outside, NAM_contra) was performed by analysis of variance (ANOVA) for the normally distributed values and by median test for the not normally distributed values. A non-parametrical Spearman Rho correlation coefficient was calculated for the metabolites and the Ki-67 MIB marker. A Bonferroni-Holm correction was performed for multiple testing.
Results
Seventeen of 26 patients had CSI data. Eight had a tumor recurrence, eight remained stable, and in 10 patients follow-up data were not available. Twenty-four patients had a GBM; patients # 20 and # 22 had a gliosarcoma. An overview of patient data is given in Table 1.
Table 1.
Clinical data, image reading and histopathology.
| Pat. no | Sex | Age | Anatomical localization | Recurrence | Necrosis | Edema/Infiltration | Mass effect | CSI | IDH | Ki-67 (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 40 | Right insula, temporal | Yes | Pronounced | Edema | Mod/Sev | No | Neg | 30 |
| 2 | F | 73 | Left temporal | ND | Pronounced | Edema | Mild | Yes | Neg | 30 |
| 3 | M | 73 | Frontal | ND | Pronounced | Edema | Mild | Yes | Pos | 30 |
| 4 | F | 37 | Frontal | ND | Pronounced | Infiltration | Mod/Sev | Yes | Neg | 60 |
| 5 | M | 49 | Right temporal | Yes | Pronounced | Edema | Mod/Sev | No | Neg | 15 |
| 6 | F | 81 | Left insula, temporal | ND | Pronounced | Infiltration | Mild | Yes | Neg | 30 |
| 7 | F | 62 | Frontal | Yes | Minor | Edema | Mild | Yes | Neg | 20 |
| 8 | F | 71 | Parietal | No | Pronounced | Edema | Mod/Sev | Yes | Neg | 10 |
| 9 | M | 66 | Frontal | ND | Pronounced | Infiltration | Mod/Sev | No | Neg | 30 |
| 10 | F | 82 | Frontal | ND | Pronounced | Edema | Mod/Sev | No | Neg | 10 |
| 11 | F | 60 | Parietal | No | Pronounced | Edema | Mild | Yes | Neg | 15 |
| 12 | M | 71 | Frontal | Yes | Pronounced | Infiltration | Mod/Sev | Yes | Neg | 15 |
| 13 | M | 64 | Occipital | ND | Pronounced | Edema | Mild | No | Neg | 20 |
| 14 | M | 73 | Frontal | ND | Pronounced | Infiltration | Mild | No | Neg | 40 |
| 15 | M | 76 | Occipital | No | Pronounced | Edema | Mild | Yes | Neg | 20 |
| 16 | M | 53 | Left temporal | Yes | Pronounced | Edema | Mild | Yes | Neg | 40 |
| 17 | M | 76 | Left temporal | Yes | Pronounced | Edema | Mild | No | Neg | 30 |
| 18* | F | 48 | Frontal | ND | Pronounced | Edema | Mild | Yes | Pos | 30 |
| 19 | M | 65 | Occipital | Yes | Pronounced | Edema | Mild | Yes | Neg | 50 |
| 20 | F | 59 | Right insula, temporal | No | Pronounced | Infiltration | Mild | Yes | Neg | 30 |
| 21 | M | 62 | Right temporal | Yes | Minor | Infiltration | Mild | Yes | Neg | 60 |
| 22 | M | 41 | Frontal | No | Pronounced | Edema | Mod/Sev | Yes | Neg | 30 |
| 23 | F | 32 | Parietal | No | Minor | Infiltration | Mild | Yes | Pos | 25 |
| 24 | F | 55 | Left temporal | No | Minor | Infiltration | Mod/Sev | Yes | Pos | 20 |
| 25 | F | 50 | Left insula | ND | Minor | Infiltration | Mild | No | Neg | 15 |
| 26 | F | 57 | Parietal | No | Pronounced | Infiltration | Mod/Sev | No | Neg | 10 |
CSI: chemical shift imaging; F: female; IDH: isocitrate dehydrogenase; M: male; Mod: moderate; ND: no data; Neg: negative; Pat. no.: patient number; Pos: positive; Sev: severe.
Radiophenomic classification
Necrosis-related module
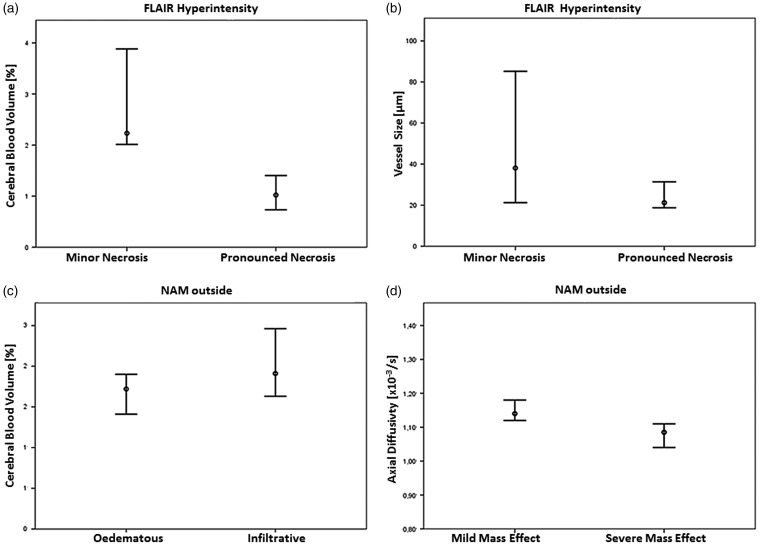
Twenty-one patients were classified as having pronounced necrosis, five as having minor necrosis. CBV in flair_hyper was higher in minor necrosis (median ± SD, 2.23% ± 0.93) than in pronounced necrosis (1.02% ± 0.71), pcorr = 0.0003, Figures 1(a) and 2(a). In flair_edge a similar result was found with higher CBV in minor necrosis (2.12% ± 0.68) than in pronounced necrosis (1.24% ± 0.48), pcorr = 0.0006, not shown. An observation of our study that unfortunately did not reach significance is that the VS (estimated by perfusion imaging) tends to be larger in minor than in pronounced necrosis (puncorr = 0.049; pcorr = 0.2) in flair_hyper, Figure 2(b).
Figure 2.
Mesoscopic measures plotted against radiophenomic modules.
(a) Necrosis-related module: Median ± 2 SD of CBV in FLAIR hyperintensity is higher in minor necrosis than in pronounced necrosis, pcorr = 0.0003.
(b) Median ± 2 SD of vessel size in FLAIR hyperintensity is higher and has a larger variance in minor necrosis, pcorr = 0.2.
(c) Edema-related module: Median ± 2 SD of CBV in ipsilateral normal-appearing matter (NAM) outside of T2/FLAIR hyperintense tumor is lower in edema than in infiltration, pcorr = 0.039.
(d) Proliferation-related module: Median ± 2 SD of axial diffusivity ipsilateral normal-appearing matter (NAM) outside of T2/FLAIR hyperintense tumor is lower in moderate to severe mass effect than in mild or no mass effect, pcorr = 0.048.
CBV: cerebral blood volume; FLAIR: fluid-attenuated inversion recovery.
Edema/Infiltration-related module
Fifteen patients were classified as having edema, 11 as having infiltration. CBV in NAM_outside was lower in edema (median ± SD, 1.72% ± 0.31) than in infiltration (1.91% ± 0.35), pcorr = 0.039, Figure 1(b) and 2(c).
Mass effect-related module
Sixteen patients had mild or no mass effect, 10 had moderate to severe mass effect. AD in NAM_outside was lower in the group with moderate to severe mass effect (median ± SD, 1.08 *10–3 mm2/s ± 0.08) than in the group with mild or no mass effect (1.14 *10–3 mm2/s ± 0.06), pcorr = 0.048, Figure 1(c) and 2(d).
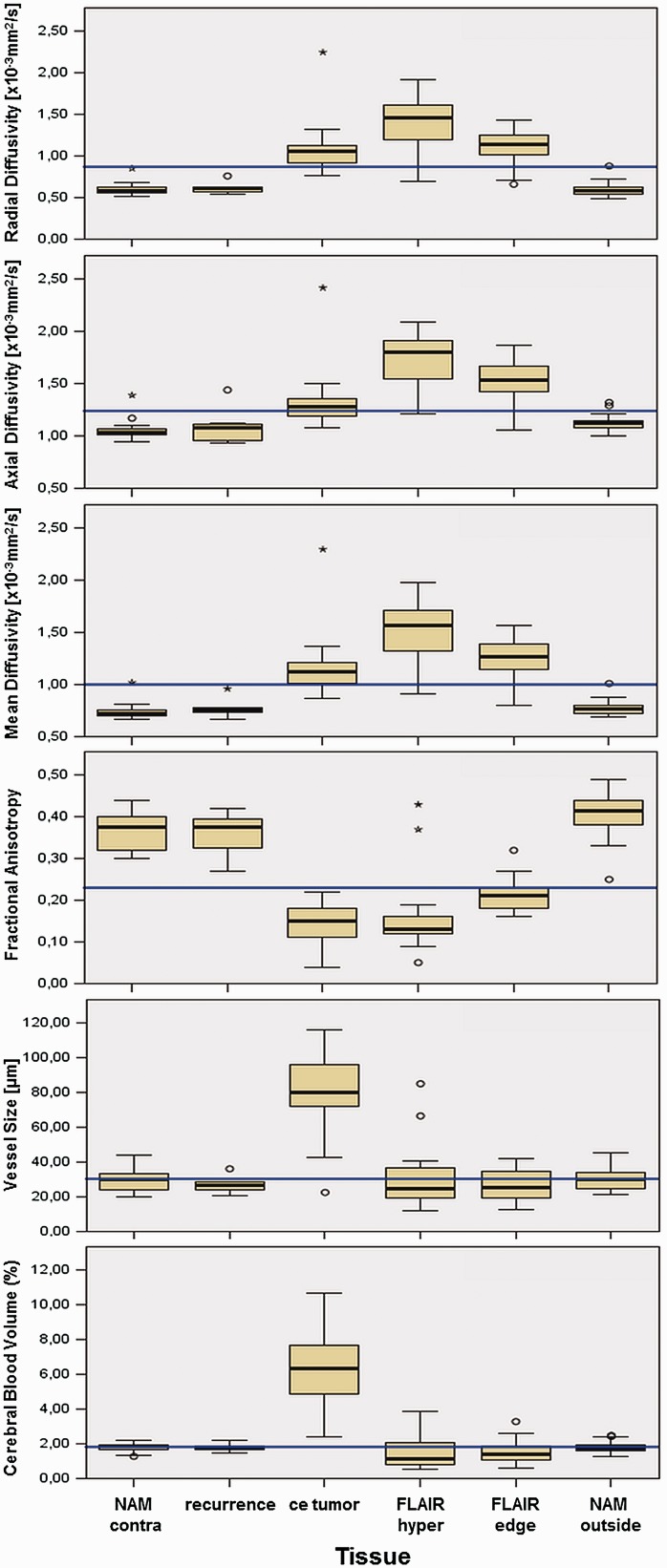
For the other mesoscopic measures and for metabolites from CSI, no significant differences were found according to the radiophenomic classification. For an overview, the diffusion and perfusion measures were sorted by ROI and displayed as whisker plots, Figure 3.
Figure 3.
Mesoscopic measures over investigated tissues.
Displayed from top to bottom are the median ± 2 SD of radial, axial and mean diffusivity (×10–3 mm2/s), fractional anisotropy (dimensionless), vessel size (µm), and CBV (%).
Displayed from left to right are the different investigated ROIs: contralateral normal-appearing matter (NAM_contra), recurrence, contrast-enhancing tumor (ce_tumour), T2/FLAIR hyperintense non-contrast-enhancing tumor (flair_hyper), non-contrast-enhancing tumor adjacent to the contrast enhancement (flair_edge), and normal-appearing matter adjacent but outside the tumor edge (NAM_outside). The total median of each mesoscopic measure is indicated in blue.
CBV: cerebral blood volume; FLAIR: fluid-attenuated inversion recovery.
Beyond radiophenomic classification
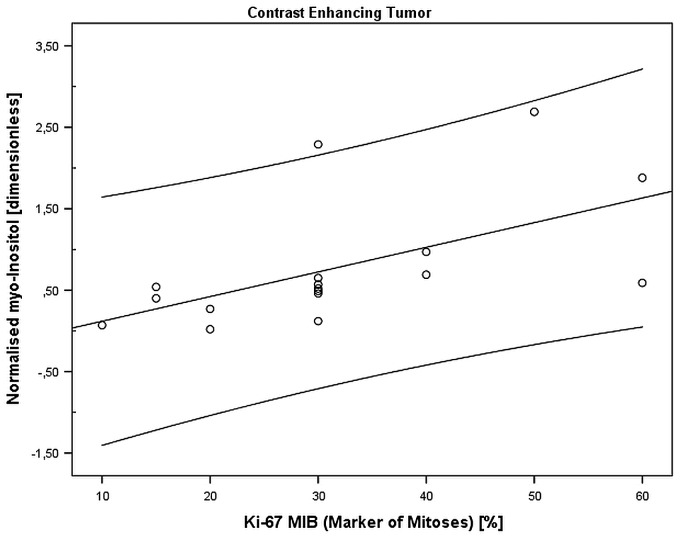
Spectroscopy revealed that in ce_tumour, nmI was positively correlated with the marker for mitosis Ki-67, r = 0.5, pcorr = 0.0002, Figure 4. It is important to note that nmI is the ratio of myo-inositol of the contrast-enhancing tumor to contralateral NAM. This means that at a level of nmI = 1, myo-inositol of enhancing tumor is equal to contralateral NAM, and that there is a spectrum of tumors with lower and higher myo-inositol compared to contralateral NAM. Neither Cho nor any other metabolite correlated with Ki-67.
Figure 4.
Correlation between myo-inositol and Ki-67 (MIB-1).
In the contrast-enhancing tumor, myo-inositol (normalized to contralateral NAM) was correlated with the mitosis rate as assessed by Ki-67 (MIB-1) (in %), r = 0.5, pcorr = 0.0002.
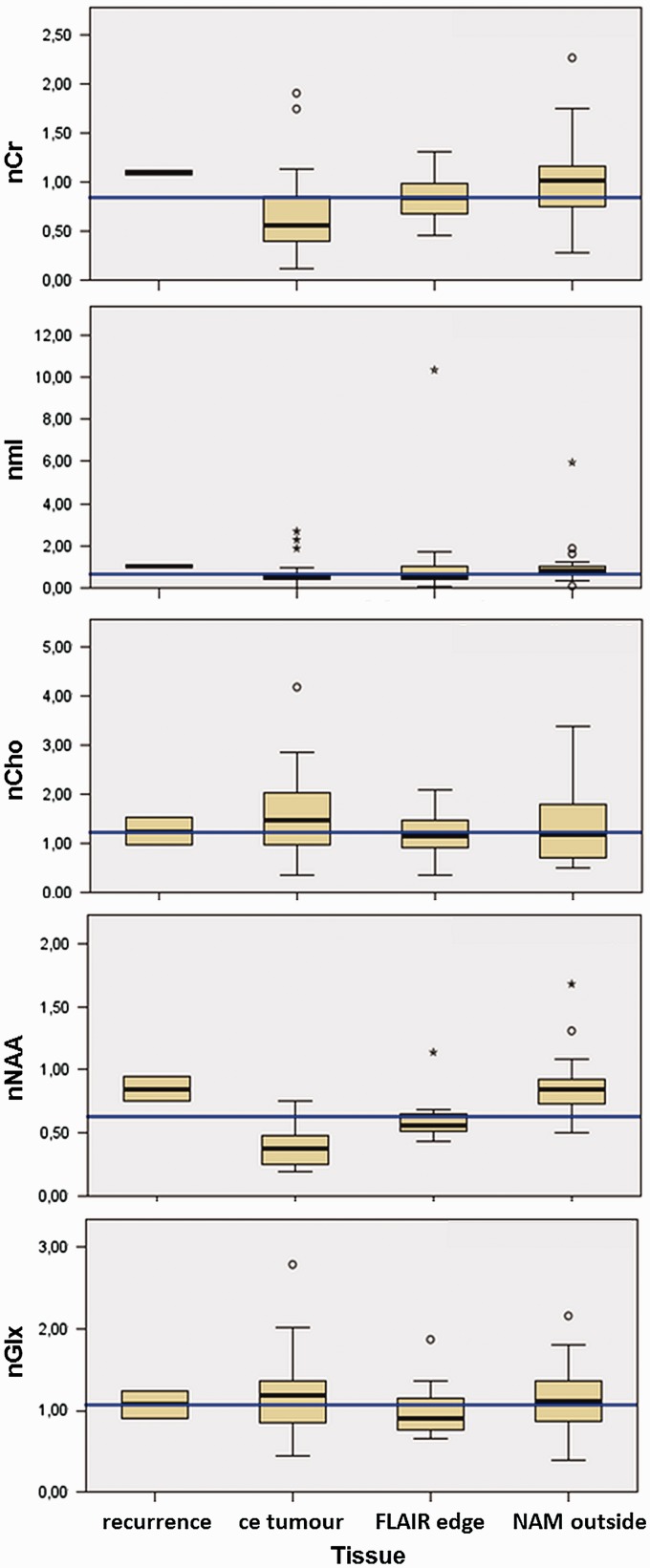
Further, a tendency for nCr of all ROIs (ce_tumour, flair_hyper, NAM_outside) to be lower and for Glx to be higher in the presence of an IDH1 mutation as compared to IDH wild type was observed. In the presence of an IDH1 mutation, nCr was 0.30 ± 0.37 (median ± SD, n = 5 spectra of two patients) and nGlx was 1.23 ± 0.76 (median ± SD, n = 5 spectra of two patients). In IDH wild type, nCr was 0.85 ± 0.44 (median ± SD, n = 43 spectra of 15 patients), puncorr = 0.049, and nGlx was 1.03 ± 0.44 (median ± SD, n = 43 spectra of 15 patients), puncorr = 0.045. These results, however, did not withstand correction for multiple testing. For an overview, the metabolites were sorted by ROI and displayed as a whisker plot, Figure 5. In addition, the metabolite values sorted by ROI are given in the Supplementary Table S2.
Figure 5.
Metabolites over investigated tissues.
Displayed from top to bottom are the median ± 2 SD of nCr, nmI, nCho, nNAA and nGlx, each normalized to contralateral normal-appearing matter (NAM).
Displayed from left to right are the different investigated ROIs: recurrence, contrast-enhancing tumor (ce_tumour), non-contrast-enhancing tumor adjacent to the contrast enhancement (FLAIR edge), and normal-appearing matter adjacent but outside the tumor edge (NAM_outside). Indicated is the total median of each metabolite in blue.
Cr: creatine; nCr: Crispi/Crcontra; Cho: choline-containing compounds; nCho: Choipsi/Chocontra; nNAA: N-acetylaspartate; mI: myo-inositol; Glx: sum of glutamine and glutamate; nGlx: Glxipsi/Glxcontra; ROIs: regions of interest; FLAIR: fluid-attenuated inversion recovery.
For recurrent tumor, neither diffusion or perfusion imaging nor MR spectroscopy yielded significant differences between the area of recurrence and NAM_outside and NAM_contra at time point 0.
Discussion
With the release of the revised World Health Organization (WHO) classification of brain tumors,26 the genetic characterization of gliomas is brought to the fore, thus leading to an increased interest in highlighting connections between the genetic profile of glioblastomas and MRI characteristics. This has already been carried out for structural MRI7–11 and perfusion-weighted imaging.12–14 The focus of this work was on the use of mesoscopic measures as surrogate markers for specific gene expression patterns in GBM.
Necrosis-related module
In the work of Diehn et al., high contrast to necrosis ratio was correlated to EGFR overexpression.2 Also, Gupta et al. showed that CBV was higher in a group of GBMs with EGFR mutation (CBV: 3.31%) than without EGFR mutation (CBV: 2.62%).14 Our findings of a higher CBV in the group with minor necrosis seem to be in agreement with these reports.
Especially the EGFR variant III (EGFRvIII) induces tumor angiogenesis with a higher microvessel density in GBMs via angiopoietin like 4 protein (Angptl4).27 An interesting additional, albeit not significant, observation of our study is that the VS (estimated by perfusion imaging) tends to be larger in minor than in pronounced necrosis (pcorr = 0.2). As the VS correlates with the capillary size in histopathology,22 smaller CBV and smaller VS in the group with pronounced necrosis could be interpreted as an insufficient adaptation of the vessels upon tumor growth.
Edema/Infiltration-related module
In comparison to patients having an infiltrative T2-hyperintensity pattern, patients classified as having an edematous pattern had a lower CBV in the NAM outside of the FLAIR hyperintense region surrounding the tumor. The fact that CBV differences can be found in the NAM outside the FLAIR-hyperintense region surrounding the tumor is new. Until now, no paper differentiated between CBV values from normal appearing matter outside the T2-hyperintense region and CBV values inside the perifocal T2-hyperintense regions. A literature search from July 6, 2016, did not yield any papers reporting this finding; see link below: http://www.ncbi.nlm.nih.gov/pubmed/?term=%28%28%28%28%28%28CNS+tumour%29+OR+CNS+tumor%29%29+OR+%28%28brain+tumor%29+OR+brain+tumour%29%29%29+AND+%28%28peritumoral+normal+matter%29+OR+peritumoural+normal+matter%29%29+AND+MRI.
In the work of Diehn et al., the group with the edematous pattern was characterized, among others, by the proneural marker genes OLIG1 and OLIG2,2 and therefore seems related to the “proneural expression subgroup” described by Verhaak et al., which has a slightly better prognosis as compared to the classical subtype.28 In contrast, the patient group with the infiltrative T2-hyperintensity pattern was characterized by an upregulation of migration-associated genes including BCAN, a gene known to play a crucial role in GBM aggressiveness. The survival of these patients was worse than that of those with the edematous pattern. This allows the assumption that GBM tumors exhibiting the infiltrative radiophenotype could correspond to the more aggressive “classical expression subtype” as described by Verhaak et al.,28 which is also associated with EGFR amplification. As Van Meter et al. also found an increased expression of EGFR in the periphery of some GBMs,4 the same factors discussed for the necrosis-related module could therefore account for the alteration of CBV surrounding tumors with an infiltrative radiophenotype.
To see whether absence of necrosis and infiltrative pattern often occur together, a Chi square test between presence/absence of necrosis and infiltration/edema was performed. It did not reveal a significant distribution conspicuousness (pcorr = 0.29). A missing connection between FLAIR hyperintensity and necrosis has also been published by Zinn et al., who described a connection between the volume of FLAIR hyperintensity and upregulated genes that promote cellular migration.11
Mass effect-related module
Rao et al. showed that a combination of tumor volume, hemorrhage and T1/FLAIR volume were related to an involvement of genes reflecting proliferation and invasion in glioblastomas.10 In addition, Diehn et al. found a strong correlation between mass effect and expression of proliferation genes.2 In this study, we discovered that the peritumoral NAM exhibits significantly lower AD in patients with moderate/severe mass effect than in patients with mild or no mass effect. Therefore, one could argue that the lower AD seen in the NAM surrounding the FLAIR hyperintensity in GBM patients with moderate/severe mass effect is likely to correlate with higher expression of proliferation genes than in patients with mild or no mass effect.
In summary, the fact that different CBV and AD values were seen depending on patient assignment to separate radiophenomic modules seems to indicate that there is a possibility of addressing genetic characteristics of GBMs using mesoscopic imaging. Moreover, it is possible to find mesoscopic changes in NAM adjacent to the tumor.
Metabolites and immunohistochemistry
Myo-inositol is a marker of astrocytes and astrogliosis. In this study we found a positive correlation between myo-inositol and the Ki-67 (MIB-1) proliferation index. A link between myo-inositol and proliferation of gliomas has been reported before.29 Valverde et al. reported that myo-inositol values of C6 rat glioma cells differ between cells in the log phase (proliferating cells) and cells that have reached confluence (quiescent cells) in vitro. In contrast with our findings, however, the inositol values diminished with decreasing proliferation.29 We do not have a straightforward explanation for these discrepancies. However, the different experimental settings (in vitro rat glioma cells versus in vivo human GBM) could be partly responsible. Further, the mI/Cr concentration ratio has been reported to be diminished in higher-grade astrocytic tumors,30 tumors which usually also exhibit higher proliferation rates.31,32 Again, this seems contradictory to our result. However, in contrast to the study by Castillo et al., where mI was normalized to Cr (mI/Cr ratio), the spectroscopic measurements in our study were normalized to the contralateral NAM. This method of normalization was favored as Cr, which is often said to be constant, has actually been shown to vary depending on the glioma grade.33 This could explain why our results do not match those of other groups.
No correlation was found between Cho levels and the Ki-67 index. This is consistent with a report from Shimizu et al., who could show that Cho levels correlate well with the Ki-67 index in homogenous, lower-grade gliomas but not in GBMs having a more heterogeneous appearance than the lower-grade tumors.34
In addition, it is important to note that there were no significant differences in the metabolites of spectroscopy depending on the IDH1-R132H mutation status. 2-Hydroxyglutarate (2HG) was not assessed as 2HG measurement requires a TE of 97 ms and the TE used here was 30 ms. There was only a Bonferroni-corrected non-significant difference between nCr in IDH1 mutant and IDH wild-type tumors. The median nCr of IDH-mutant tumor (median ± SD, 0.30 ± 0.37) was less than half of that in IDH wild type (0.83 ± 0.43), puncorr = 0.04. In the literature, no information is available on whether alterations of 2HG occur together with changes of Cr. However, if one considers that 2HG accumulation is due to an IDH-mutation-induced reduction of α-ketoglutarate from the citric acid cycle,35 a link between 2HG and Cr can be hypothesized since both are players in energy metabolism. To assess this matter, further investigations on a larger cohort are necessary.
Clinical significance
The aim of radiogenomic research is to enable a prediction of the genetic properties of a tumor so as to expedite diagnosis and treatment planning. In addition, radiogenomic research may provide new insights into tumor biology.
For perfusion imaging, our results seem in agreement with findings by Diehn et al. and Gupta et al., suggesting that EGFR-status in GBM could be predicted via the amount of necrosis and CBV.2,14 Further, the smaller VS observed in patients with pronounced necrosis can be interpreted as insufficient adaptation to tumor growth. One can hypothesize that a correlation between VS and angiogenetic gene signatures exists, but this will need to be investigated more precisely in a separate study.
For diffusion imaging, we could show that AD can potentially serve as a surrogate marker to predict the activity of proliferation genes. Also MR spectroscopy showed a parameter correlating with proliferation: myo-inositol. Together, these insights could help in identifying highly proliferative tumor regions to be targeted during biopsy or to be removed during surgery.
MR spectroscopy also showed differences of the normalized Cr values depending on the IDH mutation status. IDH-mutated tumors are known to have better prognosis than wild-type cases and IDH-mutation is predictive of a better response to chemotherapy using alkylating agents.36,37 The differences found in this study could help predict the IDH-status in newly diagnosed GBMs, thereby providing early prognostic information relevant to the patient and enabling early treatment planning.
Weighting of quantified data in comparison to the literature
The metabolites normalized to the contralateral NAM were in line with the literature for ce_tumour, flair_hyper, flair_edge, and NAM_outside.38 The diffusion metrics MD (similar to ADC) and FA for all tissues were similar to the work of Deng et al.39 FA, MD, AD, and RD for tumor infiltration in the work by Min et al. were consistent with the values of flair_edge in this study.40 Values of FA, AD, and RD were within the range given for healthy white matter during aging41 and in contralateral white matter of patients with gliomas.42 The values obtained by perfusion imaging such as CBV and VS are discussed in the section “necrosis-related module” and are in line with the literature.12–14,22
Limitations
One clear limitation is the restricted genetic information of this study. As this is a retrospective case study, only the IDH1-R132H mutation state (tested by immune histochemistry, and not by IDH sequencing) was available for all patients, whereas only five patients were tested for MGMT. The missing information on genetic variability is the reason why we refrained from calculating curves for progression-free intervals. Another limitation is the small number of patients. A larger prospective study is necessary to address changes on a mesoscopic level and their dependence on genetic variability as performed for structural imaging.2,9 Also, because of the small number of IDH-mutated tumors, the metabolite differences found between IDH-mutated versus wild-type tumors need to be interpreted very carefully. Finally, one could criticize that tumor variability was accounted for by ROIs in different tissues, but not intraoperatively, where only the contrast-enhancing rim was taken as a sample. This is because of the retrospective design of the presented study.
Conclusion
Mesoscopic imaging (perfusion and diffusion-weighted imaging) shows alterations in FLAIR hyperintensity (CBV) and adjacent NAM (CBV and AD) of GBMs depending on their “radiophenomic” patterns. These are related to the radiogenomic imaging traits by Diehn et al.2 and distinguish between “presence of minor or pronounced necrosis,” “presence of edema or infiltration” and “mild or no mass effect versus moderate to severe mass effect.”
Furthermore, myo-inositol in the contrast-enhancing tumor area reflects proliferative changes as assessed by higher Ki-67 (MIB-1) indices.
Supplementary Material
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
T.L. received funding from the German Research Society DFG grant LA3353/2-1. D.H.H. received Funding by the German Cancer Society Junior Seeding Grant IIT 110536. I.M. and A.W. received funding by the Comprehensive Cancer Center Freiburg. I.M. received funding from the German Research Society DFG grant MA-2343/4-1.
References
- 1.Barajas RF, Hodgson JG, Chang JS, et al. Glioblastoma multiforme regional genetic and cellular expression patterns: Influence on anatomic and physiologic MR imaging. Radiology 2010; 254: 564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diehn M, Nardini C, Wang DS, et al. Identification of noninvasive imaging surrogates for brain tumor gene- expression modules. Proc Natl Acad Sci 2008; 105: 5213–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pope WB, Chen JH, Dong J, et al. Relationship between gene expression and enhancement in glioblastoma multiforme: Exploratory DNA microarray analysis. Radiology 2008; 249: 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Meter T, Dumur C, Hafez N, et al. Microarray analysis of MRI-defined tissue samples in glioblastoma reveals differences in regional expression of therapeutic targets. Diagn Mol Pathol Am J Surg Pathol Part B 2006; 15: 195–205. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008; 455: 1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark K, Vendt B, Smith K, et al. The Cancer Imaging Archive (TCIA): Maintaining and operating a public information repository. J Digit Imaging 2013; 26: 1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gevaert O, Mitchell LA, Achrol AS, et al. Glioblastoma multiforme: Exploratory radiogenomic analysis by using quantitative image features. Radiology 2014; 273: 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gutman DA, Cooper LAD, Hwang SN, et al. MR imaging predictors of molecular profile and survival: Multi-institutional study of the TCGA glioblastoma data set. Radiology 2013; 267: 560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jamshidi N, Diehn M, Bredel M, et al. Illuminating radiogenomic characteristics of glioblastoma multiforme through integration of MR imaging, messenger RNA expression, and DNA copy number variation. Radiology 2014; 270: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao A, Rao G, Gutman DA, et al. A combinatorial radiographic phenotype may stratify patient survival and be associated with invasion and proliferation characteristics in glioblastoma. J Neurosurg 2016; 124: 1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zinn PO, Mahajan B, Majadan B, et al. Radiogenomic mapping of edema/cellular invasion MRI-phenotypes in glioblastoma multiforme. PloS One 2011; 6: e25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain R, Poisson LM, Gutman D, et al. Outcome prediction in patients with glioblastoma by using imaging, clinical, and genomic biomarkers: Focus on the nonenhancing component of the tumor. Radiology 2014; 272: 484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain R, Poisson L, Narang J, et al. Genomic mapping and survival prediction in glioblastoma: Molecular subclassification strengthened by hemodynamic imaging biomarkers. Radiology 2013; 267: 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta A, Young RJ, Shah AD, et al. Pretreatment dynamic susceptibility contrast MRI perfusion in glioblastoma: Prediction of EGFR gene amplification. Clin Neuroradiol 2015; 25: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.RIDER NEURO MRI – The Cancer Imaging Archive (TCIA) Public Access – Cancer Imaging Archive Wiki https://wiki.cancerimagingarchive.net/display/Public/RIDER+NEURO+MRI;jsessionid=C265998B69299A086485470F5B972DB6 (accessed 2 November 2016).
- 16.Kiselev VG, Novikov DS. Transverse NMR relaxation as a probe of mesoscopic structure. Phys Rev Lett 2002; 89: 278101. [DOI] [PubMed] [Google Scholar]
- 17.Kiselev VG. Transverse relaxation effect of MRI contrast agents: A crucial issue for quantitative measurements of cerebral perfusion. J Magn Reson Imaging 2005; 22: 693–696. [DOI] [PubMed] [Google Scholar]
- 18.Kiselev VG, Strecker R, Ziyeh S, et al. Vessel size imaging in humans. Magn Reson Med 2005; 53: 553–563. [DOI] [PubMed] [Google Scholar]
- 19.Troprès I, Grimault S, Vaeth A, et al. Vessel size imaging. Magn Reson Med 2001; 45: 397–408. [DOI] [PubMed] [Google Scholar]
- 20.Novikov DS, Jensen JH, Helpern JA, et al. Revealing mesoscopic structural universality with diffusion. Proc Natl Acad Sci U S A 2014; 111: 5088–5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baslow MH. Evidence that the tri-cellular metabolism of N-acetylaspartate functions as the brain’s ‘operating system’: How NAA metabolism supports meaningful intercellular frequency-encoded communications. Amino Acids 2010; 39: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 22.Kellner E, Breyer T, Gall P, et al. MR evaluation of vessel size imaging of human gliomas: Validation by histopathology. J Magn Reson Imaging 2015; 42: 1117–1125. [DOI] [PubMed] [Google Scholar]
- 23.Zaitsev M, Hennig J, Speck O. Point spread function mapping with parallel imaging techniques and high acceleration factors: Fast, robust, and flexible method for echo-planar imaging distortion correction. Magn Reson Med 2004; 52: 1156–1166. [DOI] [PubMed] [Google Scholar]
- 24.Fibertools | Universitätsklinikum Freiburg, https://www.uniklinik-freiburg.de/mr-en/research-groups/diffperf/fibertools.html (accessed 1 July 2016).
- 25.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993; 30: 672–679. [DOI] [PubMed] [Google Scholar]
- 26.Reuss DE, Sahm F, Schrimpf D, et al. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an ‘integrated’ diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol (Berl) 2015; 129: 133–146. [DOI] [PubMed] [Google Scholar]
- 27.Katanasaka Y, Kodera Y, Kitamura Y, et al. Epidermal growth factor receptor variant type III markedly accelerates angiogenesis and tumor growth via inducing c-myc mediated angiopoietin-like 4 expression in malignant glioma. Mol Cancer 2013; 12: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010; 17: 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valverde D, Quintero MR, Candiota AP, et al. Analysis of the changes in the 1H NMR spectral pattern of perchloric acid extracts of C6 cells with growth. NMR Biomed 2006; 19: 223–230. [DOI] [PubMed] [Google Scholar]
- 30.Castillo M, Smith JK, Kwock L. Correlation of myo-inositol levels and grading of cerebral astrocytomas. AJNR Am J Neuroradiol 2000; 21: 1645–1649. [PMC free article] [PubMed] [Google Scholar]
- 31.Raghavan R, Steart PV, Weller RO. Cell proliferation patterns in the diagnosis of astrocytomas, anaplastic astrocytomas and glioblastoma multiforme: A Ki-67 study. Neuropathol Appl Neurobiol 1990; 16: 123–133. [DOI] [PubMed] [Google Scholar]
- 32.Sallinen PK, Haapasalo HK, Visakorpi T, et al. Prognostication of astrocytoma patient survival by Ki-67 (MIB-1), PCNA, and S-phase fraction using archival paraffin-embedded samples. J Pathol 1994; 174: 275–282. [DOI] [PubMed] [Google Scholar]
- 33.Mader I, Roser W, Hagberg G, et al. Proton chemical shift imaging, metabolic maps, and single voxel spectroscopy of glial brain tumors. Magma N Y N 1996; 4: 139–150. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu H, Kumabe T, Shirane R, et al. Correlation between choline level measured by proton MR spectroscopy and Ki-67 labeling index in gliomas. Am J Neuroradiol 2000; 21: 659–665. [PMC free article] [PubMed] [Google Scholar]
- 35.Xu W, Yang H, Liu Y, et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 2011; 19: 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SongTao Q, Lei Y, Si G, et al. IDH mutations predict longer survival and response to temozolomide in secondary glioblastoma. Cancer Sci 2012; 103: 269–273. [DOI] [PubMed] [Google Scholar]
- 37.Houillier C, Wang X, Kaloshi G, et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology 2010; 75: 1560–1566. [DOI] [PubMed] [Google Scholar]
- 38.Di Costanzo A, Scarabino T, Trojsi F, et al. Proton MR spectroscopy of cerebral gliomas at 3 T: Spatial heterogeneity, and tumour grade and extent. Eur Radiol 2008; 18: 1727–1735. [DOI] [PubMed] [Google Scholar]
- 39.Deng Z, Yan Y, Zhong D, et al. Quantitative analysis of glioma cell invasion by diffusion tensor imaging. J Clin Neurosci 2010; 17: 1530–1536. [DOI] [PubMed] [Google Scholar]
- 40.Min Z, Niu C, Rana N, et al. Differentiation of pure vasogenic edema and tumor-infiltrated edema in patients with peritumoral edema by analyzing the relationship of axial and radial diffusivities on 3.0T MRI. Clin Neurol Neurosurg 2013; 115: 1366–1370. [DOI] [PubMed] [Google Scholar]
- 41.Bender AR, Raz N. Normal-appearing cerebral white matter in healthy adults: Mean change over 2 years and individual differences in change. Neurobiol Aging 2015; 36: 1834–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen-Thanh T, Reisert M, Anastasopoulos C, et al. Global tracking in human gliomas: A comparison with established tracking methods. Clin Neuroradiol 2013; 23: 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.