Abstract
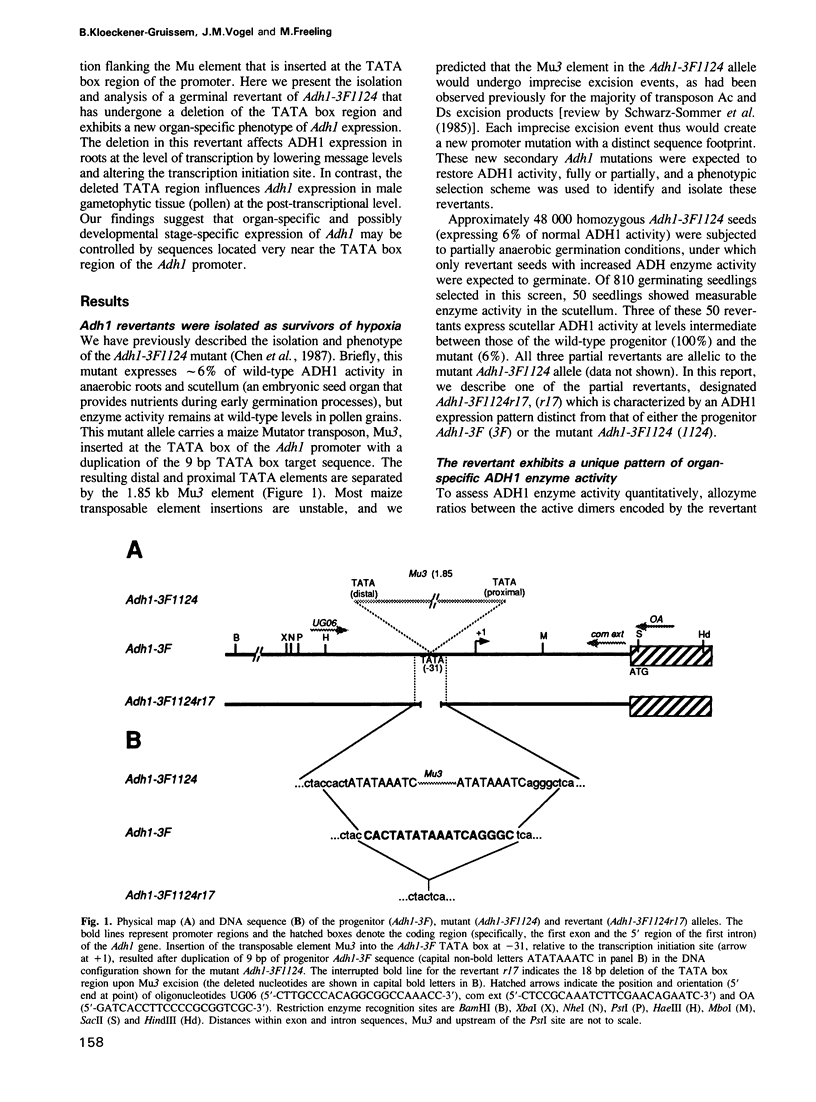
We have isolated two lineage-related Mutator (Mu3) transposon-induced Adh1 promoter mutants in maize: Adh1-3F1124 carries a duplicated TATA box and its revertant, Adh1-3F1124r17, bears a deleted TATA box. Both alterations lead to unique patterns of organ-specific ADH1 enzyme expression. Enzyme activity in Adh1-3F1124 sporophytic organs (scutellum and roots) is greatly reduced, while activity levels remain normal in the male gametophyte (pollen). Conversely, enzyme activity in Adh1-3F1124r17 roots and scutellum is partially restored, but is concomitantly reduced in pollen. Transcript analysis suggests (i) that the TATA box region of the Adh1 gene influences post-transcriptional processes in the male gametophyte but not in roots and (ii) that organ-specific transcription signals in the promoter are distinct from the previously identified anaerobic environment-specific cis-acting transcription signals. Different organs appear to provide surrogate TATA function in different ways, leading to organ-specific differences in the length of the Adh1 message 5' leader.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Chen C. H., Oishi K. K., Kloeckener-Gruissem B., Freeling M. Organ-specific expression of maize Adh1 is altered after a Mu transposon insertion. Genetics. 1987 Jul;116(3):469–477. doi: 10.1093/genetics/116.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Struhl K. Saturation mutagenesis of a yeast his3 "TATA element": genetic evidence for a specific TATA-binding protein. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2691–2695. doi: 10.1073/pnas.85.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Struhl K. Yeast upstream activator protein GCN4 can stimulate transcription when its binding site replaces the TATA element. EMBO J. 1989 Jan;8(1):261–268. doi: 10.1002/j.1460-2075.1989.tb03372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concino M. F., Lee R. F., Merryweather J. P., Weinmann R. The adenovirus major late promoter TATA box and initiation site are both necessary for transcription in vitro. Nucleic Acids Res. 1984 Oct 11;12(19):7423–7433. doi: 10.1093/nar/12.19.7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff N., Wessler S., Shure M. Isolation of the transposable maize controlling elements Ac and Ds. Cell. 1983 Nov;35(1):235–242. doi: 10.1016/0092-8674(83)90226-x. [DOI] [PubMed] [Google Scholar]
- Ferl R. J. ARF-B(2): A Protein Complex that Specifically Binds to Part of the Anaerobic Response Element of Maize Adh 1. Plant Physiol. 1990 Jul;93(3):1094–1101. doi: 10.1104/pp.93.3.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeling M., Bennett D. C. Maize Adh1. Annu Rev Genet. 1985;19:297–323. doi: 10.1146/annurev.ge.19.120185.001501. [DOI] [PubMed] [Google Scholar]
- Freeling M. Spontaneous forward mutation versus reversion frequencies for maize Adh1 in pollen. Nature. 1977 May 12;267(5607):154–156. doi: 10.1038/267154a0. [DOI] [PubMed] [Google Scholar]
- Garrity P. A., Wold B. J. Tissue-specific expression from a compound TATA-dependent and TATA-independent promoter. Mol Cell Biol. 1990 Nov;10(11):5646–5654. doi: 10.1128/mcb.10.11.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch A., Hoffmann A., Horikoshi M., Roeder R. G., Chua N. H. Arabidopsis thaliana contains two genes for TFIID. Nature. 1990 Jul 26;346(6282):390–394. doi: 10.1038/346390a0. [DOI] [PubMed] [Google Scholar]
- Gerlach W. L., Pryor A. J., Dennis E. S., Ferl R. J., Sachs M. M., Peacock W. J. cDNA cloning and induction of the alcohol dehydrogenase gene (Adh1) of maize. Proc Natl Acad Sci U S A. 1982 May;79(9):2981–2985. doi: 10.1073/pnas.79.9.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. K., Lebowitz P., Frisque R. J., Gluzman Y. Identification of a promoter component involved in positioning the 5' termini of simian virus 40 early mRNAs. Proc Natl Acad Sci U S A. 1981 Jan;78(1):100–104. doi: 10.1073/pnas.78.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangrande A., Mettling C., Martin M., Ruiz C., Richards G. Drosophila Sgs3 TATA: effects of point mutations on expression in vivo and protein binding in vitro with staged nuclear extracts. EMBO J. 1989 Nov;8(11):3459–3466. doi: 10.1002/j.1460-2075.1989.tb08510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein U., England B., Tjian R. Characterization of Drosophila transcription factors that activate the tandem promoters of the alcohol dehydrogenase gene. Cell. 1985 Jul;41(3):965–977. doi: 10.1016/s0092-8674(85)80077-5. [DOI] [PubMed] [Google Scholar]
- Hirsh J., Morgan B. A., Scholnick S. B. Delimiting regulatory sequences of the Drosophila melanogaster Ddc gene. Mol Cell Biol. 1986 Dec;6(12):4548–4557. doi: 10.1128/mcb.6.12.4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S. L., Manley J. L. DNA sequence required for initiation of transcription in vitro from the major late promoter of adenovirus 2. Proc Natl Acad Sci U S A. 1981 Feb;78(2):820–824. doi: 10.1073/pnas.78.2.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S., Xu Y. H., Stratton R. H., Roe B. A., Merlino G. T., Pastan I. Characterization and sequence of the promoter region of the human epidermal growth factor receptor gene. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4920–4924. doi: 10.1073/pnas.82.15.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi C. P. An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res. 1987 Aug 25;15(16):6643–6653. doi: 10.1093/nar/15.16.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin B. Commitment and activation at pol II promoters: a tail of protein-protein interactions. Cell. 1990 Jun 29;61(7):1161–1164. doi: 10.1016/0092-8674(90)90675-5. [DOI] [PubMed] [Google Scholar]
- Mathis D. J., Chambon P. The SV40 early region TATA box is required for accurate in vitro initiation of transcription. Nature. 1981 Mar 26;290(5804):310–315. doi: 10.1038/290310a0. [DOI] [PubMed] [Google Scholar]
- McKnight S. L. Functional relationships between transcriptional control signals of the thymidine kinase gene of herpes simplex virus. Cell. 1982 Dec;31(2 Pt 1):355–365. doi: 10.1016/0092-8674(82)90129-5. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. W., McEwan C., McKie A. B., Reid A. M. Expression of the mouse HPRT gene: deletional analysis of the promoter region of an X-chromosome linked housekeeping gene. Cell. 1986 Jan 31;44(2):319–328. doi: 10.1016/0092-8674(86)90766-x. [DOI] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posakony J. W., Fischer J. A., Maniatis T. Identification of DNA sequences required for the regulation of Drosophila alcohol dehydrogenase gene expression. Cold Spring Harb Symp Quant Biol. 1985;50:515–520. doi: 10.1101/sqb.1985.050.01.063. [DOI] [PubMed] [Google Scholar]
- Pugh B. F., Tjian R. Mechanism of transcriptional activation by Sp1: evidence for coactivators. Cell. 1990 Jun 29;61(7):1187–1197. doi: 10.1016/0092-8674(90)90683-6. [DOI] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Gierl A., Cuypers H., Peterson P. A., Saedler H. Plant transposable elements generate the DNA sequence diversity needed in evolution. EMBO J. 1985 Mar;4(3):591–597. doi: 10.1002/j.1460-2075.1985.tb03671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. C., Fisch T. M., Benecke B. J., Nevins J. R., Heintz N. Definition of multiple, functionally distinct TATA elements, one of which is a target in the hsp70 promoter for E1A regulation. Cell. 1988 Mar 11;52(5):723–729. doi: 10.1016/0092-8674(88)90410-2. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Schmidt M. C., Berk A. J., Baltimore D. Transcriptional activation by Sp1 as directed through TATA or initiator: specific requirement for mammalian transcription factor IID. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4509–4513. doi: 10.1073/pnas.87.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer H., Bonas U., Saedler H. Transposon-induced alterations in the promoter region affect transcription of the chalcone synthase gene of Antirrhinum majus. Mol Gen Genet. 1988 Jan;211(1):49–55. doi: 10.1007/BF00338392. [DOI] [PubMed] [Google Scholar]
- Thomson A. A., Ham J., Bakker O., Parker M. G. The progesterone receptor can regulate transcription in the absence of a functional TATA box element. J Biol Chem. 1990 Oct 5;265(28):16709–16712. [PubMed] [Google Scholar]
- Walker J. C., Howard E. A., Dennis E. S., Peacock W. J. DNA sequences required for anaerobic expression of the maize alcohol dehydrogenase 1 gene. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6624–6628. doi: 10.1073/pnas.84.19.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Derbyshire R., Guy A., Molko D., Roget A., Téoule R., Chambon P. Specific in vitro transcription of conalbumin gene is drastically decreased by single-point mutation in T-A-T-A box homology sequence. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7024–7028. doi: 10.1073/pnas.77.12.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefald F. C., Devlin B. H., Williams R. S. Functional heterogeneity of mammalian TATA-box sequences revealed by interaction with a cell-specific enhancer. Nature. 1990 Mar 15;344(6263):260–262. doi: 10.1038/344260a0. [DOI] [PubMed] [Google Scholar]
- Woodman J. C., Freeling M. Identification of a genetic element that controls the organ-specific expression of adh1 in maize. Genetics. 1981 Jun;98(2):357–378. doi: 10.1093/genetics/98.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]







