Abstract
In recent years, there has been increasing concern over the possibility of a radiological or nuclear incident occurring somewhere in the world. Intelligence agencies frequently report that terrorist groups and rogue nations are seeking to obtain radiological or nuclear weapons of mass destruction. In addition, there exists the real possibility that safety of nuclear power reactors could be compromised by natural (such as the tsunami and subsequent Fukushima accident in Japan in March, 2011) or accidental (Three Mile Island, 1979 and Chernobyl, 1986) events. Although progress has been made by governments around the world to prepare for these events, including the stockpiling of radiation countermeasures, there are still challenges concerning care of patients injured during a radiation incident. Because the deleterious and pathological effects of radiation are so broad, it is desirable to identify medical countermeasures that can have a beneficial impact on several tissues and organ systems. Cellular therapies have the potential to impact recovery and tissue/organ regeneration for both early and late complications of radiation exposure. These therapies, which could include stem or blood progenitor cells, mesenchymal stromal cells (MSCs) or cells derived from other tissues (e.g., endothelium or placenta), have shown great promise in treating other nonradiation injuries to and diseases of the bone marrow, skin, gastrointestinal tract, brain, lung and heart. To explore the potential use of these therapies in the treatment of victims after acute radiation exposure, the National Institute of Allergy and Infectious Diseases cosponsored an international workshop in July, 2015 in Paris, France with the Institut de Radioprotection et de Sûreté Nucléaire. The workshop included discussions of data available from testing in preclinical models of radiation injury to different organs, logistics associated with the practical use of cellular therapies for a mass casualty incident, as well as international regulatory requirements for authorizing such drug products to be legally and readily used in such incidents. This report reviews the data presented, as well as key discussion points from the meeting.
INTRODUCTION
The United States (U.S.) and French Governments both recognize the need to support development of medical countermeasures (MCMs) to treat injuries resulting from radiological and nuclear exposures due to natural disaster, accident or attack. In France, this need was recognized through the passage of French Act No. 2001-398 of May 9, 2001 which was enacted through Order No. 2002-254 of February 22, 2002. Through this Order, the Institut de Radioprotection et de Sûreté Nucléaire (IRSN) was established and tasked with providing the French Government with expertise in nuclear and radiological risks, and related scientific and technical issues. IRSN specialties include the development of innovative MCMs for treatment of radiation injury and the support for operational medical management of such victims, as requested by the International Atomic Energy Agency (IAEA). As a complement to their research portfolio, IRSN has had direct experience in the clinical treatment of 12 cases of local cutaneous syndrome and 10 cases of hematopoietic syndrome after accidental exposures to acute radiation, through their collaboration with the Burn Treatment Centre of Percy Military Hospital (Paris, France). In the U.S., in 2004, the Department of Health and Human Services (HHS) tasked the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) with a mandate to fund research related to the development of radiation MCMs. To date, both organizations have been involved in advancing basic and translational research on the nature and treatment of injuries that occur after acute radiation exposure.
Early effects of radiation exposure – referred to as the acute radiation syndrome (ARS) – normally encompass injuries to the hematopoietic (HE) and gastrointestinal (GI) compartments of the body and manifest within days of exposure. The delayed effects of acute radiation exposure (DEARE) commonly include injuries to the lungs, skin, heart, brain and kidneys and can take many months to years to arise. Despite the broad potential offered by cellular therapies, currently, the U.S. Food and Drug Administration (FDA) has not approved any such treatments for ARS/DEARE. However, the IRSN, together with the Percy Hospital in France, has been at the forefront of using these cellular approaches to treat radiation injuries sustained during industrial and medical radiation accidents, especially injuries to the skin. To better understand the potential role that cellular therapies could play in the treatment of radiation injuries from a radiological or nuclear incident, the NIAID co-sponsored a workshop with the IRSN, held in Paris, France on July 28–29, 2015, to discuss data available from testing in preclinical models of radiation injury to different organs, clinical experience in the use of cellular therapies for real-world radiation injuries, as well as regulatory considerations associated with the licensure and practical use of cellular therapies for a mass casualty incident. This meeting involved the participation of stem cell experts from the U.S. and Europe, who were brought together to address relevant questions associated with the use of cellular therapies to treat radiation-induced injuries to different organs, in the context of their potential use in a mass casualty incident. In addition to reviewing available literature for the use of cellular therapies as a mitigator of normal tissue radiation injury, this report includes data and discussion points from the meeting.
BACKGROUND
Definition and Sources of Stem Cells
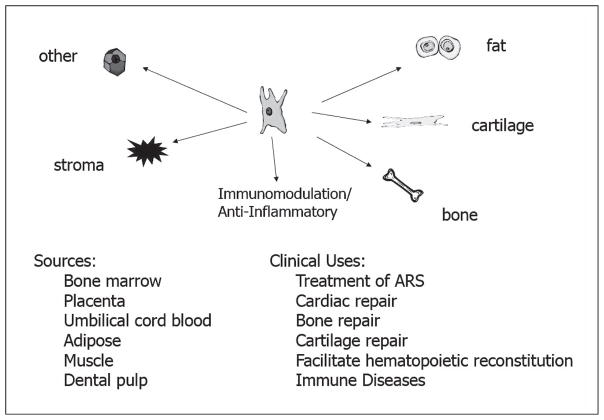
There are three types of stem cells: totipotent (that give rise to any cell type or a complete embryo); pluripotent [including embryonic stem cells (ESCs)]; and multipotent [including mesenchymal stem/stromal cells (MSCs)]. ESCs, MSCs and progenitor cells and have been collected from various tissues, including bone marrow, adipose tissue, placenta, amniotic fluid, cord blood, vascular endothelium and gingival/dental tissue (1). Other cells and cell sources with potential for use as cellular therapies for radiation injury include fetal-derived cells (2), induced pluripotent stem cells (iPSCs) (3), umbilical cord blood (UCB) cells (4), placental-derived stromal fractions (5) and vascular endothelial cells (6–9).
An attractive feature of MSCs is their immunosuppressive activity, which could potentially allow for allogeneic use without human leukocyte antigen (HLA) matching or the need for immunosuppression. A recent editorial and review explored MSC clinical uses, specifically their potential use as a medical countermeasure in a military setting (10, 11). The authors noted that MSCs, in response to chemo-attractants, home to injury sites in the body, where they then differentiate into specific tissue types, proliferate (replacing injured tissue) and secrete numerous anti-inflammatory factors to facilitate repair in endogenous tissues (12). Differentiation and paracrine signaling have both been implicated as mechanisms by which MSCs improve tissue repair.
MSCs have shown great flexibility and represent an important cellular therapy with implications for treatment of radiation injuries. For example, Osiris Therapeutics (www.osiris.com) has studied a mesenchymal cell product (Prochymal®) for use in a radiation incident. These bone marrow-derived MSCs yield benefits in cartilage repair (13), chronic obstructive pulmonary disease (14) and graft vs. host disease (GvHD) (15). In addition, MSCs from cord blood units, which do not need to be fully HLA-matched to be effective (16), have shown improved survival in models of hematopoietic and GI-ARS (17–23). Human UCB cells have been shown to successfully engraft in irradiated mice (18) and reconstitute circulating blood counts (24), and co-transplantation of stromal cells from cord blood units along with hematopoietic stem cells has been shown to further promote reconstitution (25). Human UCB transplants have also been used for successful transient rescue of hematopoiesis in an accident victim (26). As an extension to cord blood-derived cells, MSCs derived from the placenta are considered “immunologically-privileged”, and are not subject to removal by the immune system, giving them a substantial advantage for use in a mass casualty scenario, as they can be used “off the shelf”. One example is PLX-R18, a placentally-derived cellular product, which is being evaluated by Pluristem Therapeutics, Inc. in a phase I clinical trial to treat incomplete hematopoietic recovery after hematopoietic cell transplantation. These cells release factors that can improve bone marrow function, and have shown efficacy in minimizing ischemic damage (27), and as mitigators of radiation injury (5). Finally, in murine studies, adipose tissue-derived MSCs improved survival and reconstitution of multiple hematopoietic lineages when administered after radiation exposure (28). As presented in the studies outlined above, stem cells derived from many tissues have shown potential for use as treatments for radiation injury. However, to advance the development of a cellular therapy for amelioration of radiation injury, it is important to understand how the therapeutic could be used in humans, and to have a defined path forward for its licensure.
Clinical use of Cellular Therapies
Although the meeting was focused on the potential use of cellular therapies to treat radiation injury, their routine clinical use to treat other diseases has provided important clinical and safety information on their potential use during a radiation mass casualty incident. Perhaps most well-known is the use of stem cell transplants for the treatment of cancers, such as leukemia (29), lymphoma (30) and multiple myeloma (31) or to treat diseases such as aplastic or sickle cell anemia (32) or other congenital conditions that affect the bone marrow. The stem cells administered in many of these cases have been bone marrow- (autologous or allogeneic) and/or cord blood-derived. Many of these stem cell treatments involved a priori radiation and/or chemotherapy preparative regimens. In addition, the application of cellular therapies to other non-cancer indications, some of which are already licensed, include GvHD (33, 34), stroke (35), inflammatory bowel disease (36) lung disease (37), lupus (38), myocardial repair (39) and recovery from ischemia [e.g., limb (40, 41) and renal (42, 43)]. Although this list is by no means complete, information obtained from these studies can be leveraged to better understand the safety attributes of stem cells and how they could be used clinically for a radiation injury. A discussion on available preclinical data, as well as the clinical use of cellular therapies for radiation-induced injuries can be found in the meeting program overview below.
MEETING PROGRAM OVERVIEW4
The workshop brought together U.S. and European experts from the stem cell biology, radiation biology, and regulatory fields to address several questions associated with the use of stem cell therapies to treat different manifestations of radiation syndrome. A listing of all presenters and discussants is shown in Table 1. Talks were divided into several sessions. These included: regulatory and logistical challenges; consideration of cellular therapies for radiation injuries to different organ systems (cutaneous, hematopoietic, GI and other tissues); and company presentations. An overview of the presentations by, and discussions among, the meeting participants during these sessions is provided. Where scientific opinions (and/or pre-publication data) are mentioned, they are referenced by the last name of the participant.
TABLE 1.
Workshop Speakers/Discussants and Areas of Expertise*
| Name | Affiliation (at time of meeting) | Area of expertise |
|---|---|---|
| Theresa Allio, PhD | ORA, NIAID, NIH, Rockville, MD | U.S. and international regulatory affairs |
| Steven Bauer, PhD | Cell and Tissue Therapy Branch, CBER, FDA, Bethesda, MD | U.S. regulatory affairs, mesenchymal stem cells |
| Marc Benderitter, PhD | IRSN, Fontenay-aux-Roses, France | Radiation injury to skin, GI, BM, cell therapy, radiobiology, epidemiology |
| Jean-Marc Bertho, PhD | IRSN, Fontenay-aux-Roses, France | Radiation injury, immunology, hematology |
| Rob Coppes, PhD | University Medical Center, Groningen, The Netherlands | Radiation of lung and salivary glands, purification/characterization of stem/progenitor cells; xerostomia |
| Christine Czarniecki, PhD | ORA, NIAID, NIH, Rockville, MD | U.S. and international regulatory affairs |
| Andrea DiCarlo, PhD | RNCP, NIAID, NIH, Rockville, MD | Radiobiology, product development |
| Jacques Galipeau, MD | Emory University, Atlanta, GA | Cell therapies, clinical trials, cryopreservation |
| Chandan Guha, MD | Montefiore Medical Center, NY | Radiation injury, gastroenterology |
| Jean-Rene Jourdain, PharmD, PhD | IRSN, Fontenay-aux-Roses, France | Public health, radiation internal contamination, dosimetry, radionuclide biokinetics, radiobiology, pharmacy |
| Charles Limoli, PhD | University of California, Irvine, CA | Radiation biology, central nervous system injury |
| Peter van Luijk, PhD | University Medical Center, Groningen, The Netherlands | Radiobiology, radiation oncology, prevention of normal tissue injuries in cancer |
| Francesca Macchiarini, PhD | RNCP, NIAID, NIH, Rockville, MD | Immunology, radiation biology, aging |
| Jeremy Magalon, PharmD | Conception University Hospital, Marseille, France | Cell therapy, adipose-derived stem cells, stromal vascular fraction, EU regulations |
| Bert Maidment, PhD | RNCP, NIAID, NIH, Rockville, MD | Product development, pharmacology, radiobiology, pathology |
| Ram Mandalam, PhD | Cellerant Therapeutics, San Carlos, CA | Cell therapies for degenerative diseases and cancer; ex vivo expansion of human stem and progenitor cells |
| William McBride, PhD | University of California, Los Angeles, CA | Radiobiology, mitigators/treatments for injury |
| Maria Moroni, PhD | Armed Forces Radiobiology Research Institute (AFRRI), Bethesda, MD | Minipig models of radiation injury (hematopoietic, GI) |
| Lena Pinzur, MSc | Pluristem, Haifa, Israel | Placentally-derived mesenchymal stromal cells |
| Panagiota Sotiropoulou, PhD | Université Libre de Bruxelles, Belgium | Mechanisms of genome maintenance in skin and cancer stem cells |
| Jeremie Arash Rafii Tabrizi, MD, PhD | Weill Cornell Medical College, Qatar | Stem cell biology, gynecologic oncology, interaction of MSCs and cancer cells in chemo-resistance |
| Mohi Rezvani, PhD | University of Oxford, London, England | Radiation-induced oral mucositis and nephropathy |
| Miria Ricchetti, PhD | Pasteur Institute, Paris, France | Contributions of DNA repair in proliferation and differentiation of muscle stem cells. |
| Carmen Rios, PhD | RNCP, NIAID, NIH, Rockville, MD | Radiobiology, stem cell biology |
| Shahragim Tajbakhsh, PhD | Pasteur Institute, Paris, France | Skeletal muscle stem cells, embryonic and postnatal development, establishment, regeneration, & aging of the tissue |
| Radia Tamarat, PhD | IRSN, Fontenay-aux-Roses, France | Radiation injury to the skin, normal tissue protection, cell and radiobiology |
| Jacqueline Williams, PhD | University of Rochester, Rochester, NY | Radiation injury to the lungs, animal models and countermeasures testing |
Workshop participants had an opportunity to review this meeting report prior to journal submission
Regulatory and Logistical Challenges
There exist multiple logistical, scientific and regulatory challenges in the potential use of cellular therapies after a radiation public health emergency. These include: 1. relevance of available U.S., European and International Conference on Harmonization (ICH) regulatory guidance for use of stem cell therapies in radiation injuries; 2. availability of human stem cell therapies for preclinical testing and feasibility of their use in real-life scenarios; 3. consideration of manufacturing issues, including product release criteria and stability of stem cell products; and 4. feasibility of use of stem cell therapies for radiation injuries resulting from a radiological or nuclear mass casualty incident (small- or large-scale use). Critical requirements for registration of a therapeutic agent for a proposed clinical indication by a country’s regulatory health authority include demonstration of safety and efficacy in a clinical indication as well as quality of manufactured product quality and consistency of manufacturing. In all cases, products are reviewed on a case by case basis to see if they satisfy the regulatory agency’s requirements in each area. This section focuses on how safety and efficacy are determined for an indication related to ARS/DEARE, and the regulatory consultation and approval pathways that are available for cellular therapies targeting these indications.
In the U.S., cells and tissues that are manipulated extensively, combined with nontissue components, or are to be used for other than their normal functions would be regulated as biologics or devices requiring premarket approval by FDA. Metabolic cells and tissues, unless minimally manipulated and used for their natural function in close relatives of the person from whom they were obtained, also would be regulated as biologics requiring premarket approval by FDA.
In the U.S. and Europe, treatment of humans exposed to radiation in a public health emergency with a cell product that is not approved for such use would be considered “investigational use” and would require authorization for emergency use from the appropriate regulatory authority (FDA in the U.S.; European Medicines Agency (EMA) in Europe). The FDA authorization would be accomplished through either an investigational new drug application (IND) or an Emergency Use Authorization (EUA), while an Advanced Therapy Medicinal Product (ATMP) approval for a specific application from the EMA would be needed in Europe (44–46). Obtaining prior regulatory agency approval would facilitate the availability of such therapies, allowing U.S. and European populations to better prepare for a possible radiological or nuclear emergency.
The U.S. FDA’s regulations concerning the approval of new drugs and biologics (including cellular therapeutics) when human efficacy studies are not ethical or field trials are not feasible are codified in the FDA Animal Rule (21 CFR 314.600 through 314.650 for drugs and 21 CFR 601.90 through 601.95 for biological products). Approval under the Animal Rule can be pursued if human efficacy studies are not feasible, although evaluations of safety must still be conducted under the existing requirements for new drugs, requiring some degree of human testing (Allio). The Animal Rule states that the FDA will rely on animal studies to provide the substantial evidence of efficacy [as defined in the U.S. Food Drug and Cosmetic Act (Public Law 75–717, 52 STAT 1040)] needed for approval. It is important to note that approval under the Animal Rule pathway also carries with it post-marketing requirements, including one to conduct field studies to verify and describe the drug’s clinical benefit and to assess its safety when used as indicated when such studies are feasible and ethical. The EMA offers a parallel marketing authorization pathway, known as Marketing Authorization under Exceptional Circumstances (Article 14 (8) of Regulation (EC) No 726/2004).
The FDA and EMA provide current thinking on what data are needed to support regulatory approval under this pathway through guidance documents and consultation. The information gained from these sources is not legally binding, but are strong indications of what the agencies consider when evaluating the risk/benefit of a specific product for a proposed use. The FDA approval pathway is described in the U.S. FDA Guidance for Industry: “Product Development Under the Animal Rule” (47). As described in that guidance, for drugs developed to ameliorate or prevent serious or life-threatening conditions caused by exposure to lethal or permanently disabling toxic substances, the FDA may grant marketing approval based on adequate and well-controlled animal efficacy studies, when the results of those studies establish that the drug is reasonably likely to produce clinical benefit in humans. Drugs evaluated for efficacy under the Animal Rule should be evaluated for safety under the existing requirements for establishing the safety of new drugs, which requires human testing for safety. The Animal Rule states that FDA will rely on evidence from animal studies to provide substantial evidence [as defined in the U.S. Food Drug and Cosmetic Act (Public Law 75–717, 52 STAT 1040)] of effectiveness only when all the following four criteria are met:
There is a reasonably well-understood pathophysiological mechanism of the toxicity of the substance and its prevention or substantial reduction by the product;
The effect is demonstrated in more than one animal species expected to react with a response predictive for humans, unless the effect is demonstrated in a single animal species that represents a sufficiently well-characterized animal model for predicting the response in humans;
The animal study endpoint is clearly related to the desired benefit in humans, generally the enhancement of survival or prevention of major morbidity; and
The data or information on the kinetics and pharmaco-dynamics of the product or other relevant data or information, in animals and humans, allows selection of an effective dose in humans.
The same Guidance also addresses specific considerations for cellular therapies and advises the following:
Selection of relevant animal species for evaluation of a cellular therapy product should include consideration of the host animal’s response to the product and interactions of the cells with the host.
In vivo cell fate following delivery using the clinical route of administration should be characterized in each species.
If the cell fate, cell function, and/or host response to the cells in the animal species differs greatly from what is known or predicted in humans, administration of a well-characterized analogous cellular product in the animal studies may be considered.
The use of an analogous cellular product in an animal efficacy study is predicated on the ability to identify, harvest, and characterize (e.g., phenotyping and potency) a similar cell population in the animal species used for testing. Production of the analogous cellular product should meet the same standards as those applied to production of the final human cellular therapy product.
The FDA and EMA have both issued several additional guidance documents specific to cellular therapy development that are useful resources when one is considering development of cellular therapies. U.S. FDA guidance documents that are specific to cellular therapies include “Guidance for Industry: Preclinical Assessment of Investigational Cellular and Gene Therapy Products” (48); draft Guidance on “Homologous Use of Human Cells, Tissues, and Cellular and Tissue-Based Products” (49); and draft Guidance “Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) from Adipose Tissue: Regulatory Considerations” (50). FDA regulations also offer intensive consultation programs for drugs intended to treat life-threatening and severely debilitating illnesses (21 CFR 312 Subpart E). Under the FDA regulations, the task of giving scientific advice is assigned to FDA staff, who may then consult with additional internal and external experts (51).
In a similar fashion, the European Union (EU) has in place a regulatory framework to approve new cellular therapies for use in human populations (45). The objectives of the ATMP regulations are to promote market access of cellular products, while ensuring protection of patients (Magalon). As in the U.S., standards of current good manufacturing processes (cGMP), good laboratory practice (cGLP) and good clinical practice (cGCP) are applicable (52). Cells based therapies are defined as ATMPs if they fulfill at least one the following criteria:
Substantial manipulation: The cells have been manipulated during the manufacturing process so that their biological characteristics, physiological functions or structural properties have been modified to be relevant for their intended function (e.g., cell expansion or genetic modification and/or differentiation or activation with growth factors).
Different essential function (non-homologous use): Cells harvested and separated by a simple selection method, and re-administered to fulfill their same essential function will generally be regarded as homologous use.
The Committee for Advanced Therapies (CAT) was established by the EMA in 2007, to more closely define cellular products that could be used clinically. The CAT is a multi-disciplinary group of European subject matter experts charged with assessing the safety, quality and efficacy of ATMPs and monitoring recent research developments. Thus, the ATMP regulations through the formation of the CAT were developed to ensure that cell therapy applications received feedback from cell therapy experts, to promote market access to cellular products while ensuring protection of patients.
There have been several phase I human clinical studies using cellular therapies for nonradiation indications in the Marseille University Hospital Cell and Culture Department. For example, in 2015, orphan designation (EU/3/15/1464) was granted by the EU to the Marseille University hospital to treat hand sclerosis using autologous, adipose-derived, stromal vascular fraction regenerative cells (ADRCs), obtained using the medical device “Celution System” produced by Cytori Therapeutics (www.cytori.com). For this indication, collection of cell samples and their expansion, transport, preparation, cryopreservation, shipment to site of use and subsequent injection have all been important considerations and are primary logistical challenges of their clinical use. Use of the ADRCs has also improved outcomes in patients with systemic sclerosis (53–56) and improved healing of thermal burns (57). Cytori Therapeutics is developing ADRCs for thermal burn injury, including burns that are complicated by radiation exposure, and these ADRCs improved healing of full thickness burns in minipigs exposed to a sub-lethal dose of total body irradiation (TBI) – a radiation combined injury (58).
As for all drug products, by the time of licensure application, validated assays must be developed and used to establish and characterize product identity and quality, including potency, purity and stability. Although validated assays are not required for trials conducted under U.S. Investigational New Drug (IND) Applications, assay validation is expected to take place during or prior to initiation of phase III clinical trials, and must be finished before licensure (Bauer). It is important to establish stability of the product under optimal storage and shipping conditions. Cellular products present unique challenges to such testing, storage and shipping and these were discussed. How the cells will be used (e.g., dose, route and in what populations), safety and efficacy must be demonstrated to provide necessary regulatory information. Objectives for preclinical development programs that are evaluating cellular therapies for radiation injury should include establishment of biological plausibility; (e.g., rationale for proposed efficacy of the specific product for the radiation indication) and identification of biologically active dose levels; selection of a starting dose (e.g., cells/kg body weight) and considerations of single vs. multiple dosing; establishment of a proposed route of administration (e.g., intravenous, intramuscular); and identification of physiologic parameters to guide clinical monitoring and consideration of potential public health risks.
Other developmental activities might involve establishment of in vitro functional assays, such as immunophenotyping and morphologic evaluations. An important component of licensure of a cellular therapy for a radiation indication is the determination that the selected animal model and evaluation are appropriately representative of the human response both to the injury and to the treatment. This would include conducting in vivo pilot and pivotal efficacy studies in the animal model and comparison to available human data – activities that would provide both proof of concept for the therapy as well as supportive data on its mechanisms of action.
Scientists employed by the FDA Center for Biologics Evaluation and Research (CBER) are tasked with consideration of licensure requests in the U.S. for cellular therapies for a radiation mass casualty indication. In addition to their review responsibilities, CBER scientists have research responsibilities, and some are active participants in an FDA MSC research consortium. The U.S. FDAs MSC consortium’s primary goal is assuring the safety and efficacy of stem cell-based products (59). This consortium hopes to develop strategies for improved identity and potency assays that could be used to predict positive clinical treatment outcomes (60). MSCs are known to be multi-potent and can differentiate to form stromal, adipose, bone or cartilage cells. They are immunosuppressive and thus can be used in the allogenic setting. Their clinical use is currently being studied for cardiac, bone, cartilage and marrow repair, as well as in the treatment of immune diseases. However, there are challenges involved in the translation of MSC therapies from preclinical studies to clinical trials (59) due to the diversity of the cells themselves, as well as how they are produced and their site of origin. It is important to understand how these factors can influence performance of these cells for a clinical indication.
MSC characterization, studies being undertaken by the U.S. FDA MSC Consortium include genomics (61), epigenetics (62), proteomics (63), and single cell PCR, as well as studies on models of ischemia, in vitro differentiation (64) and immunosuppression (65). MSCs from donors of different gender and age backgrounds, were expanded then frozen at different passage numbers. The resultant MSCs vary in their ability to expand, as well as their size, colony forming units and differentiation into different cell types. Several important publications (referenced above) document the plasticity and potential use of these adaptable cells.
MSCs have frequently been studied for treatment of GvHD (33, 66, 67). Using a universal donor approach for treatment of patients with these cells, several approaches proved to be unsuccessful in the clinic. For example, Prochymal, an MSC stromal cell product developed by Osiris Therapeutics, did not meet its primary clinical endpoint for GvHD (68), and multipotent adult progenitor cells (Multistem®) developed by Athersys also did not meet the primary clinical endpoint for stroke (improvement in stroke recovery at 90 days) or colitis, despite showing promise in a preclinical model of ischemic injury (69). To better understand why these studies were not successful, it is important to examine factors such as donor variability, immunogenicity, expansion complications, and freeze/thaw protocols. Functional determinants predict that MSC immune suppressive potency can vary markedly between healthy random donors, which may bias efficacy of a “universal product” (70).
In some transplant settings, it is possible to use fresh cells, which, although preferred, would be challenging during a radiation public health emergency. Freezing cells may alter their biological characteristics and potency; furthermore, thawing and immediately infusing such a cellular product may not allow for full recovery of original identity and potency. For example, it has been demonstrated that frozen and thawed MSCs have impaired interferon-gamma responses (71), although 48 h of culture after thawing rescues function (72). This is likely because structural integrity of cells can be temporarily compromised post-thaw. In addition, frozen and thawed MSCs are sensitized to lysis by complement (73). The finding that fresh cells are more effective than freeze/thawed cells has been shown clinically for treatment of GvHD patients, in which fresh MSCs yielded a better patient response (73). Another consideration in terms of influence on proliferation includes the isolation method employed (74). Several methods to augment MSC function prior to transfusion to improve outcomes are currently being studied (Galipeau).
Cellular Therapies for Cutaneous and Muscle Injuries
Optimum healing of a cutaneous wound requires a well-orchestrated integration of the complex biological and molecular events of cell migration and proliferation, extracellular matrix (ECM) deposition, angiogenesis and remodeling. Moreover, immune and stem cell mobilization and tissue neovascularization are important aspects of skin regeneration. Resident stem cells, as well as terminally differentiated cells, home to a lesion, where they secrete factors that can be curative, but also can lead to overt inflammatory responses resulting in scarring and tissue fibrosis (Tamarat). Administration of exogenous stem cells may stimulate neovascularization – with the cells capable of differentiating into endothelial or smooth muscle cells. The cells can also induce paracrine growth factors and vasodilators, which can contribute to healing, a finding that is further supported by the observation that MSC-conditioned medium also enhances tissue repair (75). To date, MSC treatment of acute and chronic wounds has resulted in accelerated wound closure with increased epithelialization, granulation tissue formation and angiogenesis. Although there is evidence for MSC differentiation in the wound, most of the therapeutic effects are likely due to MSCs releasing soluble factors that regulate local cellular responses to cutaneous injury.
There are several stem cells in residence in the skin epidermis. These include inter-follicular, sebaceous and hair follicle bulge stem cells. Sebaceous gland stem cells are sensitive to both high and low radiation doses. In sharp contrast, bulge stem cells appear to be resistant to radiation-induced cell death, both at high and low doses, albeit employing distinct mechanisms to achieve this resistance (76, 77). Interestingly, the differential response of bulge and sebaceous gland stem cells to low-dose radiation arises, even though the level of DNA damage is comparable in the two stem cell types. Moreover, age has been shown to modify epidermal stem cell responses to low-dose radiation (LDR), sensitizing bulge stem cells to LDR (Sotiropoulou). Reconstituting affected areas with single donor, bone marrow-derived MSCs has previously been shown to impact radiation induced skin fibrosis by altering the chronic inflammation set up by the initial exposure (78, 79). In the clinic, radiation exposures from radiology procedures have also been shown to cause DNA damage within skin cells (80).
Importantly, the most advanced use of cellular therapies has been in the treatment of radiation-induced skin complications. This advancement is the result of a collaboration between radio-pathologists and physicians from the IRSN, in support of clinicians from Percy Hospital (Centre de Transfusion Sanguine des Armées), who have demonstrated the efficacy of specific MSC therapies in the treatment of patients with radiation cutaneous injuries from industrial accidents. In addition, there have been several preclinical models of radiation-induced skin injury that demonstrated the efficacy of MSCs (both endogenous and exogenously administered) in suppressing inflammation and reducing fibrosis and scarring (78, 81–85). It is worth noting that there are significant differences between radiological and thermal burns in terms of clinical symptoms and evolution. This explains why the medical management of severe radiation burns after accidental overexposure to ionizing radiation remains a highly challenging therapeutic issue. The medical management of severe radiation burns after accidental overexposure to ionizing radiation is still a major therapeutic challenge, since such injuries are generally not resolved using the classical therapeutic approaches derived from the management of thermal or electrical burns.
Several industrial accidents have resulted in patients developing severe skin lesions. These types of injuries are especially challenging to address, as radiological burns have been observed to have successive inflammatory waves of injury (86, 87). For example, after a radiation accident in the Republic of Georgia, one patient required five skin autografts. The healing process was drawn out, and the outcome was, at times, uncertain (88). Another industrial accident in Yanango, Peru (1999) led to severe cutaneous radiation injuries, when a worker placed an unshielded Ir-192 source in his back pocket (89). The patient was treated at Percy Military Hospital in France, but the severity of the injury at the time of hospital admission led to a disarticulation of the hip after failed xenografts. The first therapeutic approach was based on a “wait and see”, i.e., wait the maximum amount of time before intervention. Unfortunately, this led to an extension of the lesion and was responsible for the disarticulation.
Recent advances have improved outcomes. In a more recent radiological accident in Chile in 2005, a worker unknowingly handled and placed a radiation source in his back pocket. The estimate of the worker’s radiation exposure was 1,600 Gy to the left buttock, as well as other cutaneous injuries. Thus, the victim sustained multiple lesions leading to ulceration and underlying tissue injuries, and was transferred to the Hospital d’Instruction des Armées Percy (Paris, France) for treatment. Among the therapies administered to the patient was local injection of bone marrow-derived, autologous MSCs. This cellular therapy was associated with a reduction in pain, inhibition of the progression of the lesion, and ultimately, complete closure of the largest lesion within 90 days (90). Another accident occurred in Senegal in 2007 (85), in which the victim had radiation-induced bone marrow injuries, as well as skin complications. In addition to receiving cytokines to address the hematopoietic myelosuppression, the patient also received MSCs delivered as multiple, local injections at the site of the burn. Administration of MSCs led to complete healing of the skin, as well as lowered pain and improved function. The underlying muscle and bone that were also damaged by the exposure improved as well.
In support of human clinical evidence of the efficacy of cellular therapies, preclinical work on several models of radiation-induced injuries has been carried out, which suggest that these approaches might also be successful in treating thermal burn and/or wounds whose healing is complicated by total or partial-body radiation exposure (radiation combined injury). In an early preclinical study, adipocyte-derived stem cells were studied for their ability to improve wound healing in irradiated animals (91). Mice were given a full-thickness wound on the back, and then irradiated locally (20 Gy); wound closure rates, cutaneous flow, and immunohistochemistry were all evaluated. It was demonstrated that a local injection of stem cells accelerated the healing process by stimulating an increase in the vascular density in the wound bed. More importantly, green fluorescent protein (GFP)-labeled, CD-31+, adipose-derived stem cells (ADSC) were incorporated and differentiated into endothelial vascular cells (91). Similarly, ADSC have a beneficial effect on the wound healing process through their capacity to stimulate re-epithelialization associated with an increase in collagen secretion, which improves the quality of the skin by increasing its viscoelasticity (Tamarat).
In another murine model of cutaneous radiation injury, one leg was irradiated, which induced skin burns within a few weeks (1). The administration of MSCs improved wound healing in a dose-dependent manner, and multiple doses were found to be better than a single dose. Furthermore, researchers investigating the contribution of the bone marrow to the healing of skin wounds developed a model in which the radiation depth-dose distribution of led to the skin only being irradiated, versus the entire body. In that study, wound tensile strength was used as a measure to determine the patency of the repair of a skin wound in an irradiated mouse model. This nonlethal, TBI was shown to compromise wound healing (92). Although several cell types homed to the damaged tissue, they were not effectively retained in the wound; therefore, a treatment was developed utilizing fibrin microbead scaffolding, onto which MSCs were bound. The technology was further adapted to large wounds by binding the cells to collagen sheets. Application of the beads directly to the site of injury allowed them to persist and proliferate within the wound. This treatment was able to reduce the wound healing deficits from TBI, but not skin-only irradiation (92).
When there is skin exposure, as in an industrial accident, often underlying muscle is also affected. For this reason, it is reasonable to investigate radiation responses in muscle cells. It is known that dormancy and response to stress exposes distinct muscle stem cell states (93–95). In the adult muscle niche, stem cell regulation involves laminin and integrins (Tajbakhsh). Muscle stem (satellite) cells are normally quiescent, serially transplantable, and divide both symmetrically and asymmetrically. These cells are generally resistant to stress and their repair of DNA double-strand breaks (DSB) is highly efficient and accurate after irradiation (95). There are important lessons to be learned from studying muscle stem cells, which are not normally a major concern during a radiation mass casualty incident (due to their relative radioresistance). These findings include reports of active paracrine signaling during skeletal muscle regeneration, and that for fibroblasts, endothelial cells and satellite cells, transient senescence is important in tissue remodeling. In addition, the discovery that adhesion cues and niche topology guide asymmetric cell divisions and fates provide valuable insight, which is important for the study of other types of stem cells.
Cellular Therapies for Hematopoietic Injury
The first known use of cellular therapy for treatment of accidental radiation exposure occurred in 1958 at the Curie Institute in Paris, France (96). At that time, victims from an experimental nuclear reactor accident in Vinca, Yugoslavia were treated with bone marrow transplants (BMT) after being exposed to radiation. Clinical data from the accident concluded that the bone marrow provided probably led to temporary engraftment of the cells, but because it was likely administered too late after the incident, did not lead to a dramatic change in patient outcomes (96). After the Chernobyl accident in April, 1986, 13 patients were given cell transplants (13 BMT; 6 fetal liver) with a 10% survival rate (97). The following year, the Goiânia accident in Brazil led to exposure of 249 people, with 20 individuals requiring hospitalization. Eight patients were diagnosed with ARS, but received no BMT, instead receiving treatment with recombinant human granulocyte-macrophage colony stimulating factor (GM-CSF) (98). Interestingly, 50% of those patients survived, suggesting that cytokine therapy was beneficial in the treatment of ARS in the absence of a transplant. In the evaluation of several clinical case studies, where 58 patients received high doses of radiation, 29 patients received a stem cell transplant whereas the others did not. There did not appear to be a significant survival benefit from the administration of cellular therapies, unless the damage was limited to the hematopoietic system (99). For this reason, the use of growth factors alone was recommended for patients receiving moderate radiation exposures (100). These findings of limited efficacy of cell therapies may be because inhomogeneous radiation exposures can lead to endogenous recovery and graft rejection. In a later accident in 1999, two patients that received high dose TBI after a criticality incident in Tokai-mura, Japan received hematopoietic stem cell transplants (101). The transplants appeared to be successful in both cases; however, these patients subsequently died from multiple organ dysfunction and failure. The source of the transplant for one of the patients was an HLA-mismatched cord blood unit (26), whereas the other received a peripheral blood stem cell transplant. Taken together, these results indicate that the limited efficacy of cell therapies may be due to rejection of the transplanted allografts upon partial recovery from radiation exposure.
Endothelial cells (ECs) have been shown to play a role in the treatment of radiation-induced bone marrow injury. Exposure to high-dose radiation, in addition to causing tremendous damage in the bone marrow, causes severe injury to the vascular network, including the hematopoietic niche. Replacement of lost vascular ECs is critical to minimize further tissue damage and several animal studies have shown that ECs can restore hematopoiesis after lethal TBI (8, 9, 102, 103). Transplantation of ECs has been shown to mitigate injury in multiple organs, as ECs are involved in several activities including metabolism, inflammation and immunity. These cells exist in specialized vascular niches, with access to specific growth factors. ECs have tissue-specific signatures and serum-free cultivation of adult mouse or human endothelium is possible. Angiocrine factors produced by ECs balance self-renewal and differentiation of HSCs. EC treatments are showing promise in mitigation of radiation exposure injury in myelosuppressed animals (Rafii Tabrizi) and tissue-specific ECs have also been shown to be successful for use in organ regeneration (104).
Cellular Therapies vs. Growth Factors for Hematopoietic Injury
While there is a wealth of clinical information available demonstrating the use of cellular therapies for radiation-induced hematopoietic injuries, including their potential to treat bone marrow injury, one consideration in their use for hematopoietic rescue is whether the cells yield survival benefits greater than those observed with growth factors, such as granulocyte-colony stimulating factor (G-CSF), alone. Given that in 2015, two hematopoietic growth factors, Neupogen® and Neulasta® (Amgen, Thousand Oaks, CA), were approved by the U.S. FDA for both adult and pediatric patients to “increase survival in patients acutely exposed to myelosuppressive doses of radiation”, this consideration becomes even more important, since these licensures have enabled the formal procurement of these growth factors for the U.S. Strategic National Stockpile (SNS), without the need for an EUA for their deployment and use. This licensure was based on studies that found that administration of G-CSF to irradiated nonhuman primates (NHPs) (7.5 Gy TBI), increased survival by 38.3% over controls (105). It is worth noting that, in another study carried out in NHPs, no significant differences in recovery or other hematopoietic parameters were noted when G-CSF was administered alone after a high dose, heterogeneous radiation exposure (8.7 Gy with right arm shielding). In contrast, in that model, a combination treatment of G-CSF and autologous expanded bone marrow cells demonstrated marked hematopoietic recovery (106). This finding is consistent with what has been observed in humans (107).
To better understand the use of cytokines, administered alone or in conjunction with BMT, in the treatment of radiation injuries, two conferences were held in Europe in the early 2000s (108), followed by U.S./European consensus meeting that was convened in 2009 (109). Subject matter experts from these meetings recommended not providing BMTs initially, but suggested that they be considered if there are no signs of endogenous bone marrow recovery (107). Consensus accepted that cytokine treatments should be the first line of therapy for those radiation injuries that lead to severe myelosuppression; indeed, use of growth factors alone following accidental exposure has demonstrated reasonable success. By way of example, two industrial accidents occurred in 2006, one in Fleurus, Belgium, when a worker at a facility where medical devices were sterilized by radiation was exposed to an estimated 4.80 Gy (107), and similarly, a worker in Dakar, Senegal was exposed when an iridium source in a radiographic device did not retract (estimated 2.3 Gy exposure) (110). In both cases, the patients were treated only with cytokines, experienced a return to normal blood counts and survived without administration of a cellular-based therapy. Although questions remain as to the potential usefulness of cellular therapies to treat radiation injuries; these data suggest an important role for growth factors in any treatment paradigm.
Cellular Therapies for Gastrointestinal Injuries
There have been several radiation accidents that have resulted in exposures that were high enough to cause significant damage to the GI tract [e.g., Vinca (96), Chernobyl (97), Soreq (111), Nesvizh (112), Indiana and Tokai-mura (113)]. Based on data derived from the System for Evaluation and Archiving of Radiation Accidents Based on Case Histories (SEARCH) database (114), GI syndrome is estimated using clinical diagnoses related to diarrhea and GI bleeding (METREPOL scaling) (115). Depending on the severity of the injury (Grades 1–4), management of patients and their prognoses differ, with Grade 1 patients having the best prognosis for recovery and Grade 4 patients the least likelihood of survival (115). Treatment recommendations include prophylactic strategies such as anti-diarrheal drugs, analgesics and anti-inflammatories for lower grade exposures, and additional fluid and electrolyte replacement, antibacterial and anti-fungal therapy and maintenance of a sterile environment for highly irradiated individuals. Although there are several drugs under development to treat radiation injury to the GI tract, no country has yet approved a therapy to treat GI-ARS (116). Nonetheless, several studies have suggested that cellular therapies may be used to restore the intestinal stem cell niche after irradiation (117–119), and human MSCs have been shown to improve survival after irradiation in mice, restore intestinal structure and function, and increase epithelial cell proliferation while reducing apoptosis (120).
In animals exposed to 18 Gy total abdominal irradiation, severe damage can be seen within a few days postirradiation. However, when bone marrow-derived adherent stromal cells (BMASC), which contain mesenchymal stem cells, macrophage and endothelial progenitor cells (EPCs) were provided, there was recovery of the cryptvillus structure, as well as improved survival (117). Even use of the supernatants from BMASC cultures alone enhanced survival, although the presence of macrophages in the recipient mice was then found to be critical for survival. Factors that could be utilized to further mitigate radiation injury alongside a cellular therapy include R-spondin1 and growth factors (i.e., fibroblast, keratinocyte, hepatocyte or insulin-liker) (Guha). In addition, a myeloid progenitor cell therapy has been shown to improve survival in a mouse model of GI-ARS (121) (detailed in the “Company Presentations” section below) Promising work being carried out in the biology of the intestinal stem cell also enhance the probability that adult stem cell therapies may be useful for regeneration of the GI tract after injury (Benderitter).
To bring MSC therapies for GI-ARS to the clinic, the development plan would need to include large scale, cGMP manufacturing of product, GLP pivotal animal efficacy studies and phase 1 safety. Current clinical development in this research area in the EU includes GMP production of ATMP-MSC to reduce bleeding and diarrhea frequencies in radiation-induced colitis. For example, MSCs were used to treat radiation overdose of patients in a radiotherapy setting at the Jean Monnet Hospital in Epinal, France, which resulted in GI complications (122, 123). However, there are still many questions concerning the potential use of cellular therapies for GI (Benderitter). For example, what stem cell product (e.g., from the bone marrow, intestines, etc.) is best, what route of administration would be optimum (e.g., intravenous, intramuscular, intraluminal), and what would be the interaction with cytokines or other stem cell treatments.
Cellular Therapies for Other Tissue Injuries
Lung
Cellular approaches have shown promise in the treatment of radiation injuries to other organs. Perhaps the most well studied, late complication of acute radiation exposure is effects on the lung. After radiation exposure and a subsequent latency period, potentially lethal pneumonitis can develop, which may be followed months later by fibrosis and other pulmonary deficits For example, the Radiation Effects Research Foundation (RERF), through analyses of Life Span Study (LSS) data, has reported an increased rate of respiratory diseases observed in Hiroshima and Nagasaki atomic bomb survivors (http://www.rerf.jp/radefx/late_e/noncance.html) (124, 125). Other studies have also noted late lung complications in both the liquidators and exposed individuals living in contaminated areas around Chernobyl (126). In addition, lung complications were identified as contributing to the death of at least one of the victims of the Tokai-mura radiation accident (101). There are over 40 different types of stem and progenitor cells in the lung, in distinct niches with region-specificity (127), and cells from the bone marrow have been shown to play an important a role in lung repair (128). Several studies in animal models for lung diseases, such as acute respiratory distress syndrome (ARDS), acute lung injury and chronic obstructive pulmonary disease, have suggested the utility of MSCs, bone marrow-derived mononuclear cells, endothelial progenitor cells, and/or embryonic and/or iPsCs in providing benefit (Williams). The recruitment of endogenous and/or exogenously delivered stem cells can home to and/or participate in remodeling of injured lung.
MSCs for use in lung treatments could be derived from multiple tissues, including from cord blood, Wharton’s jelly, placenta, adipose, and lung (Williams). It has been shown that cells that are injected intravenously will home to lung, in an effect referred to as “pulmonary first-pass” (129). Of the four cell types studied: MSCs, multipotent adult progenitor cells (MAPCs), bone marrow-derived mononuclear cells (BMMC), and neural stem cells (NSC), most of the MSCs were found in the lungs, with other cells passing more readily through the organ. This suggests that larger cells are initially trapped in the lungs – a finding that may be advantageous for the use of these cell populations as a pulmonary radiation mitigator. MSCs have also been shown to engraft in the lung after TBI (130), and function in situ by expressing paracrine signals and trans-differentiating (131). In one experiment, fibroblasts (cloned from bone marrow stromal cells) were infused five weeks postirradiation, to facilitate late lung recovery in an irradiated canine model (132). In that study, endothelial-like progenitor cells were mobilized by the fibroblast treatment, improved lung function and total lung capacity in treated animals was noted; a decreased incidence of pneumonitis was apparent.
In summary, while cell therapies represent a potential treatment for radiation injury of the lung, the timing of their administration will be important. Since the development of lung injury is a late effect, more time is available to utilize the therapy. This is a positive factor, in that it may be possible to initiate therapy later after exposure and still see benefit; however, the microenvironment in the irradiated lung continues to vary, which means that timing of administration of the cellular therapy is critical. In addition, the genetics of the patient can affect efficacy, as can the irradiation protocol (e.g., total-body vs. partial-body or focused irradiation) (Williams).
Brain
Another recognized late effect is radiation-induced cognitive dysfunction. Radiation exposure can lead to both acute and long-term biological effects in the central nervous system. These effects can then manifest as impaired cognition, which results from depletion of neuronal stem cells and inhibition of neurogenesis (Limoli). For example, in patients that previously received radiation therapy for treatment of brain tumors, it has been documented that declines in executive, motor and memory skills can be pronounced (133). A research model of cranial radiation in a mouse has been developed, in which dramatic declines in numbers of neural stem and progenitor cells are produced (Limoli). Animals are provided human neuronal stem cells or microvesicles by direct grafting into the brains, and cognitive testing is done months later. These grafts led to improved performance on behavioral tasks, as well as a reduction in inflammation and retention in neuronal morphology (134). Exosome grafting has led to similar improvements (135).
Salivary gland
Another area in which significant clinical interest has been focused is the potential use of stem cells to address injury to the salivary gland, which is routinely experienced by patients after irradiation for head and neck tumors. Although the direct applicability of salivary gland stem cells for mass casualty use may be somewhat limited, there remains important information to be gleaned from a general understanding of stem cell-assisted remodeling of irradiated tissue (136–138). For example, mobilization of bone marrow derived cells using G-CSF (139), alone or in combination with stem cell factor (SCF) and Fms-related tyrosine kinase 3 (Flt3) ligand (140), improved morphology and vascularization resulting in improved glandular function, suggested to be due to bone marrow derived endothelial progenitor cells and paracrine secretion of MSCs (van Luijk). Mouse and human salivary gland cells are capable of self-renewal and efficient and rapid expansion in culture (141, 142), and in vivo, transplanted salivary gland stem cells are capable of self-renewal and can differentiate into all lineages. There is a stem cell therapy for radiation-induced xerostomia that will begin a planned phase I/II trial in 2018, which will further inform the potential clinical use of these important cells for a radiation mass casualty indication.
Company Presentations
Several companies involved in stem cell therapy development were also invited to participate in the meeting and discuss their work. Their insight into the advanced development of these approaches for a radiation indication provided an important context for the discussions concerning translation of these technologies from the laboratory to licensure.
Cellerant Therapeutics (www.cellerant.com) is developing treatments for oncology and blood-related disorders. One of their lead products, CLT-008, is a myeloid progenitor cell therapy derived from mobilized peripheral blood, pooled hematopoietic stem cells that is being evaluated in an oncology clinical trial. One of the key clinical endpoints being sought for CLT-008 is “reduction in infection during neutropenia in acute myelogenous leukemia patients”, and in early clinical trials, the product has been shown to be safe, to reduce incidence and duration of fever, and to reduce mucositis (Mandalam). Although this product is being developed primarily as a treatment for acute myeloid leukemia (AML), with ARS as a second indication, these myeloid progenitors have been shown to significantly improve survival in a mouse model irradiated at doses of up to 14 Gy; delaying administration of the cells up to 5 days postirradiation still provided a significant survival benefit after radiation doses that induce both the HE- and GI- sub-syndromes of ARS in animal models (121). Cellerant has received funding from the U.S. Government for its development as a treatment for HE-ARS, and plans to use the U.S. FDA Animal Rule licensure pathway to seek approval for the cell therapy. In keeping with FDA guidance, the company has developed animal analogs, for which the comparability of the human and animal cells must be established (Mandalam). These analogs have been developed for both mouse and monkey myeloid progenitors. Comparability testing has proved challenging, as many aspects of the cells, including surface marker expression, functionality and species-specific cell differences have needed to be addressed (Mandalam).
Pluristem Therapeutics, Inc. (http://www.pluristem.com/) uses “off-the-shelf” cells that are derived from the placenta, and cultures them to scale up production. They are evaluating their products to treat autoimmune dysfunction and degenerative states. The company has previously shown safety and efficacy of their first product, PLX-PAD in clinical trials (critical limb ischemia and muscle injury) (Pinzur). Their pipeline also includes PLX-R18, which is being studied for bone marrow indications as well as for HE-ARS. The PLX-R18 therapy is composed of placentally derived, adherent stromal cells that are mesenchymal stem cell-like and do not engraft in vivo. The company believes that the cells’ mechanism of action is via paracrine and endocrine signaling, as they do not appear to leave the site of intramuscular injection (Pinzur). Treatment with PLX-R18 has been shown to improve survival in a mouse model of radiation injury. Briefly, C3H/HeN mice exposed to 7.7 Gy TBI were given an intramuscular injection of PLX-R18 at 24 h and 5 days postirradiation. Animals receiving the cells had improved survival, and more rapid recovery of the bone marrow and blood cell lineages (5). The mechanism of action of the protection could be linked to an observed mobilization of differentiated cells into the blood in treated animals. The cells are also known to secrete factors that promote hematopoiesis in vivo (Pinzur).
The Cellerant Therapeutics and Pluristem Therapeutics products each offer potential short-term, benefits that could provide interim protection to an irradiated patient. These cell products, which do not appear to engraft in the long-term (and is not necessarily a requirement for their efficacy), can provide protection to the exposed individual until such time that their own bone marrow cells can reconstitute the blood cells. At least in part, the ability of these cell products to provide a survival benefit relies on remaining pockets of active marrow that might remain after irradiation, even at high dose levels. This is reasonable, given the inhomogeneous radiation exposures anticipated; however, if the doses are too high, it might be necessary to provide cells to the patients that can engraft and provide long-term benefit.
DISCUSSION
Following the presentations, several guided discussions allowed researchers to provide feedback on the state of the science of cellular therapies and identify gaps to U.S. and French government officials involved in funding and carrying out advanced development of cellular approaches for radiation-induced injury. U.S. Government representatives included staff from NIAID’s Radiation and Nuclear Countermeasures Program (RNCP) and Office of Regulatory Affairs (ORA), as well as scientists from the Armed Forces Radiobiology Research Institute (AFRRI) and FDA-CBER. The French government was represented by IRSN staff. Questions that were used to guide the framework of discussions during the meeting are shown in Table 2.
TABLE 2.
Open Discussion Topics
| Characterization of stem cells: |
|
|
| Type of stem cell, species (e.g., human, animal) and geographic (e.g., bone marrow, adipose, placental, cord blood, dental) source |
| Responses of human stem cells in different animal species – in vitro and in vivo |
| Morphologic, cytological and molecular properties associated with proliferation and differentiation state of injected stem cells in different models |
| Cell culture and use: isolation procedures, culture conditions, pre- activation modalities, cryopreservation |
| Assessment of degree of plasticity of the stem cells (trans- differentiation) |
|
|
| Mechanism of stem cell-related actions and preclinical efficacy models for radiation injury: |
|
|
| Recruitment, incorporation, division (symmetric/asymmetric), differentiation of defined stem cells in the targeted pathological tissue |
| Evaluation of putative paracrine capacity of specific stem cells |
| Determination of stem cell biodistribution, tracking, biokinetics, and fate |
| Mechanism of action of stem cells in different organ systems (e.g., bone marrow, GI tract, lung, skin) after radiation injury |
|
|
| Translational applications: |
|
|
| Assessment of stem cell routes of administration |
| Identification of functional assays (in vitro and/or ex vivo) to predict therapeutic potential |
| Estimation of stem cell efficiency by preventive or curative application. |
| Possibility to use fresh or frozen cells |
| Evaluation of allogeneic and/or autologous cell therapy approaches |
| Potentiation of stem cell therapeutic effect by pretreatment of cells (drugs or hypoxia) and/or by co-administration with biomaterials (hydrogels, matrix) |
|
|
| Clinical use of stem cells to treat radiation injuries: |
|
|
| Standardization/optimization of stem cell culture protocols for clinical application |
| Evaluation of side effects of stem cells administration (biodistribution, toxicity, etc.) |
| Stem cell banking for therapeutic use in patients |
| Ability to predict potency of cell products |
| Differential potency from different donors based on age, gender, pre-existing conditions, etc. |
|
|
| Licensure and other challenges for use of cell therapies in a radiation incident |
|
|
| Availability of human cell therapies for preclinical testing; barriers/challenges involved in collection and distribution of cells for use in real-world scenarios |
| Relevance of available U.S., European and ICH regulatory guidance for use of stem cell therapies in radiation injuries |
| Manufacturing release criteria for stem cell use, as opposed to assessment of potency (process monitoring) |
| Feasibility of use of stem cell therapies for radiation injury resulting from a radiological or nuclear, mass casualty incident (small scale, or large-scale use) |
Clinical use of Stem Cells to Treat Radiation Injuries
Meeting participants were asked to consider the challenges and possible clinical use of stem cells to treat radiation injuries. The discussion initially centered on the ways in which age, gender and microenvironment may affect the potency and differentiation potential of a cellular product. Although using a limited sample size (8 donors), the FDA MSC Consortium noted that cells from donors over the age of 40 senesced earlier than cells from younger donors, and that cells from older donors were larger in size (Bauer). Age effects were also observed when comparing stromal vascular fractions (derived from digestion of adipose tissue) from different patients (143). These studies suggested that adipose-derived stem cells were less effective as a therapy when isolated from older people versus younger donors (Rezvani). In addition, studies done in the salivary gland suggest that although there are more cells in older people, those cells do not perform as well in culture and may not be as potent (144). Once cultured, however, the cells transplant and function as well as the younger ones. This implies that potency may be affected by environmental conditions, in addition to being affected by the age of the donor (Tamarat). Similar outcomes have been observed in bone marrow cells from older patients. In a skin wound model, bone marrow cells from younger donors yielded a greater benefit on vascularization and re-epithelialization than cells derived from older donors (145). Finally, findings from Dimmeler et al. suggest that age effects in adult stem and progenitor cells can be explained, in part, by increased oxidative stress in older cells (146). In summary, there may be contributions from both the age of the transplanted cells and the tissue environment to the success of a therapy.
Comorbidity factors (e.g., hypertension, diabetes, etc.) can also influence the ability of transplanted cells to function adequately. For example, cells from the bone marrow of diabetic mice have less potential to differentiate into endothelial progenitor cells, and show a similar reduced effect on wound healing (147). In addition, in studies carried out in Buerger’s patients (a disease characterized by inflammation and thrombosis in small and medium-sized blood vessels), it was determined that stromal samples collected from these patients were less efficient at addressing critical limb ischemia than those collected from healthy patients (148). However, as was observed with the salivary gland cells, when adipocyte-derived stem cells were put into culture before administration, there was no difference between Buerger pathology and healthy patient-derived cells in terms of their use for therapeutic angiogenesis (Tamarat).
Because it is desirable to be able to characterize which stem cells will be more efficient than others, there was discussion as to what specific parameters could be assayed. To that end, it was suggested that analyzing the state of the mitochondria in cells planned for use in transplants could be used to inform on potency, since there is, in general, a direct correlation between mitochondria health and activity of the cells (Ricchetti). It was also suggested that cell size morphology could be a marker to inform stem cell potency; in the FDA MSC Consortium, the size of MSCs was observed to increase with increased passage number (Bauer). Cell proliferation can be assessed using phase contrast imaging to look at morphological parameters, and it is possible to correlate osteogenic activity to morphology. Morphological assessments can also have value in establishing cell quality for in vitro and in vivo performance. Similarly, differences have been seen comparing multiple myeloma relapse risk in the transplantation of bone marrow cells collected from male versus female donors (149). This could be due to the presence of estrogen receptors.
Characterization of Stem Cells, Mechanisms of Stem Cell-Related Actions and Preclinical Efficacy Models of Radiation Injury
In the second discussion session, conversations shifted to better understanding of the mechanism of stem cell-related actions and the value of preclinical efficacy models of radiation injury (Table 2).
Importance of animal models and role of human data
It was clear to the assembled researchers that animal models were mandatory for the testing of cellular therapies for radiation injuries, because in these models, it is possible to assess potential variables, such as irradiation characteristics (e.g., total- or partial-body irradiation), as well as evaluate combinations of injury/diseases (e.g., burn plus radiation, internal plus external radiation exposure). Animal models are also critical for establishing mechanism of action of the therapy and evidence of effectiveness; it was also acknowledged that patient populations, even those not irradiated, provide important proof-of-principle evidence that cellular therapies can be effective. Nonetheless, it is not possible to develop an animal model that entirely reflects each detail of a human victim of accidental radiation exposure. Although models exist for organ-specific and TBI injuries, in an accident, human victims will likely have heterogeneous radiation exposures, as well as local irradiation and other combined injuries. For this reason, although it is not perfect, there was agreement that the availability of radiation therapy data for countermeasure efficacy should be considered for its relevance to victims of a radiation incident. It was the belief of the participants that a cancer patient would likely be more representative of a normal healthy individual’s response to a cellular therapy than an animal’s response to the same therapy.
Although data obtained from humans is preferred, there are issues associated with utilizing information from cancer patients, in part due to the nature of their radiation exposures, focused and localized as opposed to the TBI/heterogeneous exposure expected from a radiation incident, the frequent concomitance with chemotherapy and existence of co-morbidities that could complicate analysis of their response to mitigation with a cellular therapy (Bertho). Nonetheless, it is important to learn what one can from oncology patients, since normal tissue toxicity is not determined by the cancer phenotype. For example, although a radiation therapy patient may have head and neck cancer, radiation-induced normal tissue injuries can occur in other organs not directly impacted by cancer, and can provide valuable information on the injury and its treatment. Because investigators are counseled to seek other clinical indications for their radiation medical countermeasure (Maidment), data and studies conducted for alternative indications (and subsequently tested in humans) could then possibly be leveraged to provide supportive information for the radiation indication.
There was some discussion as to the role of patient data for a radiation indication. For U.S. FDA Animal Rule licensure, the preferred primary efficacy endpoint in nonclinical animal model studies is survival; therefore, there was agreement that patient data only be used to support safety and mechanism of action of the therapy. In irradiated patients, from which samples are obtained and drugs might be tested, only parts of the body are irradiated, although there was general agreement that those irradiations are not as localized as one might be led to believe. There was consensus that if data from early clinical trials could be used to refine a preclinical model, they should be considered in licensure efforts.
In 2010, one of the first U.S. FDA approvals of a cellular therapy was for dendritic cells (Provenge®) for use in a therapeutic cancer vaccine (150). It was noted that, at the time, little information was known concerning the half-life and biodistribution of the Provenge® therapy, but its demonstrated efficacy as a cancer vaccine warranted its approval. There was some discussion as to the criticality of knowing where cells migrate to after injection. For example, if a stem cell is placed in a constrained space from which it does not migrate and it proliferates excessively, it might form ectopic tumors, which would be a safety concern. Similarly, some cell therapies such as MSCs might be rejected/removed by the immune system before homing to injured tissues sites, but if a survival advantage is demonstrated, there might still be sufficient evidence available to lead to approval.
Regarding the preclinical testing of animal cells, care should be taken in considering the utility of any data derived from xenotransplantation in immune competent animals (i.e., human cells that are tested for efficacy in competent animal models) (Galipeau). If efficacy is observed, a possible concern about those data might be that the observed effect was nonspecific. For example, nonspecific immune reactions to a large intravenous influx of nonsame cells have been noted (Galipeau). In a GvHD model, treatment with human cells led to an improvement in GvHD; however, if the cells were killed prior to transplantation, the therapy was still effective (Galipeau). This finding strongly suggested that the observed improvement was not due to the cells, but instead due to a nonspecific immune response.
Paracrine effects
There was discussion regarding whether it was necessary for a cell to interact directly with the tissue to exert an effect. Although for determination of the mechanism of action of the therapeutic product, it might be important to understand where cells go and with what tissues they interact post-administrating, their paracrine function is believed to be central to the efficacy of most cellular therapies. With regards to the ability of certain cell therapies to exert effects that extend beyond direct replacement of damaged cells, MSCs have been shown to yield a benefit in many different pathologies and situations, even though evidence suggests that the cells themselves often do not end up in the injured tissues, despite showing efficacy of the treatment. Instead, their efficacy appears to be due to a paracrine effect (the cells secrete factors that act on a distant site) or an indirect effect of stimulation of the immune response. In fact, paracrine effects are strongly supported by several studies, including: 1. data from Pluristem Therapeutics, which indicates that when PLX-R18 treatment is given intramuscularly, the product remains at the injection site, but can exert its effects elsewhere (Pinzur), 2. exosomes released by MSCs and acceleration of radiation-induced skin lesion healing in an accidental exposure (Tamarat), 3. brain tissue repair after radiotherapy exposure (Limoli), and 4. salivary gland, as mentioned above (Coppes) (151).
Considerations concerning the effect of number of cell passages prior to transplant on safety and efficacy of cellular therapies was also discussed. For example, in some countries, due to the risk of potential chromosomal aberrations, the numbers of passages allowed prior to transplant are limited. Within the testing done by the FDA MSC Consortium across eight bone marrow donors, some abnormalities were seen at passage three, but they tended to resolve as the cells were further passaged (152). In addition, the French experience has allowed for clinical application of MSCs for a passage not exceeding one, to circumvent the limits associated with cell culture.
In triage of victims of radiation exposure, treatment availability will likely be severely constrained, and physicians will dedicate those scarce resources to cases where they believe they can save patients (153). Although cell therapies might be used for those patients receiving higher doses of radiation, in cases where there is still believed to be some stem cell survival, there was discussion as to whether it might be more advantageous to simply administer identified paracrine substances to stimulate endogenous cells to proliferate, as opposed to administering the cells themselves. When cells are injected, for example, it takes a few days for them to migrate to the site of injury, and an additional few days to exert an effect. Given these lag times, it might be preferential to administer the paracrine factors themselves by direct injection, although many of these factors have yet to be identified. Additionally, exosome or extracellular vesicle (typically below 100 nm) approaches represent an attractive path forward for cellular therapies.
There is also often an issue of heterogeneity of harvested cells, not just between donors, but also within a patient. Cells obtained from a single patient and then further manipulated can demonstrate varied characteristics based on factors such as when they were isolated, their time in culture and passage number, and the fact that they have been frozen and then thawed. These factors can then alter the biodistribution of cells. For example, during the freeze thaw process, chemokine receptors important in tracking, as well as immune markers, cell adhesion and tracking molecules can be lost from the surface (McBride). These factors must be controlled to obtain and define a manufacturing process that will produce a consistent product for transplantation.
The benefits that could be conferred through cellular therapy for a radiation indication could go beyond improving survival, which is the generally preferred primary endpoint for efficacy studies. For example, concerns over cognitive deficiencies are legitimate, and can have a dramatic impact on quality of life of survivors, especially given their progressive nature (Limoli). For this reason, it would be important to determine how long a transplant could act to ameliorate these late cognitive deficits. Quality of life in lung injuries could also be proposed as major morbidities. There was some discussion concerning the role of treating nonlife threatening injuries with cellular therapies during a radiological or nuclear incident. It was noted, for example, that there are several nonlife threatening outcomes after radiation injury, such as diarrhea, nausea and vomiting, but these syndromes are survivable and not necessarily critical or progressive. Although it was acknowledged that acute, life-threatening syndromes are important to study, caution was urged in discounting certain non-transient quality of life concerns, such as neurocognitive deficits.
Use of cell therapies for treatment in a mass casualty setting
In the setting of a radiation public health emergency, there are logistical challenges involved in the use of either autologous or allogeneic cellular therapies. One potential difficulty for certain autologous therapies might be the need to pre-bank cells for transplant in advance of an incident. While this plan would not be reasonable for members of the public, the approach could potentially be implemented for first responders and military populations. Autologous transplants, where the cells are derived from the irradiated patient and then re-injected, also involves challenges, including the probability that the individual’s own cells might also have been irradiated along with the organs/tissues that need to be repaired. It has been shown, for example, that irradiated adipocytes are not as effective as a treatment as cells that were not exposed to radiation (91, 154). Poglio et al. described that subcutaneous adipose tissue, including the stem cells contained therein is sensitive to radiation. Since it is likely that if the bone marrow was ablated, necessitating a transplant, then the abdominal fat layer would also be heavily irradiated, the isolated cells would also likely be damaged or killed, and whether the cells would be fully functional is dependent on radiation dose (155). If the exposed individual has enough surviving cells, then they can be expanded and replace those lost to irradiation, functioning as well as cells from an unirradiated donor. In addition, given that many radiation exposures are heterogeneous, with some of the individual’s tissues not irradiated, it might be possible to derive cells from those locations for transplant. Finally, there is information obtained from clinical studies that can be leveraged, in which autologous marrow-derived cells were used to treat GvHD after allotransplant (discussed above). Most of these patients were irradiated and it was established that it was difficult to grow MSCs out of an irradiated patient. It was noted; however, that MSCs could be harvested from the less irradiated bone marrow area, as determined by physical dosimetry assessments (Coppes).
Translational Applications
Later in the meeting, issues concerning the translation of cell therapies to a mass casualty incident were discussed (Table 2). In vitro growth of cells in culture can often be challenging. In culture, most MSC-like cells behave similarly (Galipeau), and normally respond differently in a biodistribution assay only when they were frozen and then thawed versus fresh. The freeze-thaw effect, from which the cells must recover over several passages, is a complication, and normal cell-culture medium is often not sufficient for their recovery and continued growth. However, success has been seen growing cells in substances such as matrigel. For example, salivary gland stem cells grown in matrigel will form organoids, which is similar to their in vivo growth (156).
In discussions on the future clinical use of MSCs for any indication, and for MSC potentiation, it was the consensus that pre-activation of cells before injection will likely be the best way to ensure their efficacy. There are currently hundreds of clinical trials looking at the therapeutic potential of MSCs for many indications (Galipeau), and it was expressed that studies on the use of non-activated MSCs is close to exhausted. Therefore, it is critical to pursue research involving preconditioning of MSCs, which can be used to skew them toward having a transcriptome/secretome that is desirable for the organ system where they are intended for use. Because cryopreserved cells that are thawed take time in culture to return to a pre-cryopreserved metabolic state, it can be difficult to precondition them immediately after they are thawed. For this reason, it might be more advantageous to pre-activate the cells prior to freezing them. There was discussion emphasizing that all manipulations (preconditioning as well as post-thaw recovery steps) are all considered “product manufacturing steps” for the final cell product and must be appropriately controlled by cGMP regulations including release testing of the product before administration to the patient.
There exists strong evidence that there is a difference between the effectiveness of live/fresh and cryopreserved stem cells destined for transplant. This finding is also commonly made in other fields; however, the published literature covering cryopreservation (primarily reproductive, e.g., sperm, oocytes) is somewhat restricted to live/dead assessment, with a scarcity of work looking at functionality. The point was made that often, preclinical data showing efficacy of a cellular therapy uses fresh cells, which would likely not be possible in an actual clinical care setting. Use of freeze-thawed cells in both preclinical and clinical use is appropriate; however, care must be taken to ensure that findings with fresh cells are like those observed with frozen and then thawed cells. Finally, several questions remain unaddressed, posing an important challenge for the clinical transfer of stem cell applications (e.g., route of administration; single/repeated injections, expansion, fresh/frozen etc.). It remains important to standardize protocols dealing with isolation of cells and their in vitro culture before administration.
Challenges for Use of Cell Therapies in a Radiation Incident - Mass Casualty Logistics
In the final session of the meeting, discussion centered on the challenges involved in licensing and using cells as a treatment in a radiation scenario (Table 2). For a cellular therapy to be successful in humans, it is critical that injected cells not be removed from the circulation by the body’s own immune system. Using a cellular product comprised of cells that are matched to the patient can circumvent this problem; however, this approach represents a significant logistical challenge for their use in a mass casualty incident. Given the short window for initiation of therapy (within a few days after an incident), and the possibility of scarce resources in the aftermath of an incident, it will likely be difficult to administer a cellular therapy that requires matching. The probable need for intravenous administration by knowledgeable health care staff, requirement of liquid nitrogen storage conditions for frozen cell products, and availability of ancillary equipment and supplies to thaw and prepare the cells represent additional challenges. These factors will likely limit the use of most cellular therapies to a hospital setting. One way to address the challenge of matching is to use cellular therapies as a “stop-gap” measure. In this paradigm, the cells need not be matched at time of administration – an approach that has garnered interest in its potential use after a radiation incident. Given the known myelosuppression that occurs after irradiation, the body is essentially unable to reject the transplanted cells in the short-term; therefore, administered cells can circulate for some time and provide needed benefits to the patient. When the patient’s own immune responses have recovered, the host immune cells destroy the mismatched donor cells
Comments were also made concerning the availability of cells for use in research. Given that the processes involved in growing and expanding cells in culture are challenging, and that a typical academic laboratory might not have the funding necessary to culture cells under cGMP; having sources of ready-to-inject cells facilitates research. Although cells can be obtained from various commercial sources (e.g., Lonza, AllCells, Allosource), there is often variability in terms of how those cells grow in culture. There are government-funded repositories available; for example, the Production Assistance for Cellular Therapies (PACT) is a National Heart, Lung, and Blood Institute (NHLBI), NIH-funded resource initiative. The PACT is comprised of multiple cell processing facilities that were created to assist with cellular therapy translational research and the manufacture of cellular therapy products. (https://www.pactgroup.net). This program was contracted by the U.S. Government to do cGMP manufacturing of MSCs in support of first-in-human clinical trials that were supported by the NIH. In addition, other researchers in the field are often amenable to making their cells available for preclinical study. Meeting participants identified the Institute for Regenerative Medicine at Texas A&M University (formed by Darwin Prockop and funded by the NIH) as a primary source of cells used in their laboratories. The institute, which is also funded by the NIH, has a large bank of fully characterized MSCs that are made available for research.
E-cell France (https://www.ecellfrance.com/) is a French network infrastructure for regenerative medicine using MSCs. GMP grade MSCs, in accordance with EU regulations (advanced therapy 1394/2007), are produced in large scale for clinical use. Since its creation in 2012, E-cell France has been involved in over 20 national and European research and innovation programs in cell-based therapy. E-cell brings together state-of-the-art, ATMP production platforms, immune-monitoring and quality control platforms, research teams with expertise on translational research in cell therapy, and clinical research teams to cover phase I and II clinical studies (Benderitter). Moreover, E-cell France assists academicians and industrial groups with the implementation of their preclinical and/or clinical programs in the field of cell-based therapies. This infrastructure provides services covering the entire MSC-based production and development pipeline: MSC production (clinical batches meeting the European directive 1394/2007 on ATMP); quality control, potency tests and MSC safety testing; immuno-monitoring of MSC-treated patients (phenotypic analysis of sub-populations of immune cells) and preclinical studies.
From a mass casualty perspective, preferred therapies are those that can be administered at later times postirradiation and still show efficacy. Several cellular therapies have demonstrated attributes in nonclinical studies that make them desirable in this way. For example, as mentioned above, Cellerant’s CLT-008 product rescues irradiated animals even when administered 5–7 days postirradiation (121). The challenge to show efficacy when delivered at later times postirradiation is greater, for example, for treatment of acute radiation injuries, which often manifest in days to weeks, as opposed to late effects like lung complications that could take months to manifest. Generally, the later the tissue responds to the radiation injury, the later a therapy could be given as a mitigator and still show efficacy.
The Radiation Injury Treatment Network® (RITN), in the U.S. fills a readiness gap in case a radiological/nuclear incident occurs that requires patient access to bone marrow transplants (157). The U.S. National Marrow Donor Program, along with the U.S. Office of Naval Research and the American Society for Blood and Marrow Transplantation collaboratively developed RITN, which is comprised of medical centers with expertise in the management of bone marrow failure, stem cell donor centers and umbilical cord blood banks across the United States. The primary purpose of the network is to help coordinate medical response and evaluate and treat victims at participating centers (www.ritn.org). The European Bone Marrow Transplantation Association Nuclear Accident Committee (EBMT-NAC) holds regular workshops regarding capabilities, which allows for vital exchange of information, and important work has been done in Europe to advance the thinking about treatment of radiation casualties, especially the creation of the SEARCH database (114), as well as the advancements of MEdical TREatment ProtocOLs (METREPOL) for triage of radiation accident victims (115). METREPOL, initiated in 1997, has been instrumental in providing guidance that allows first responders to rapidly and accurately grade the severity of radiation-induced injuries in individuals accidentally exposed to radiation (Benderitter).
CONCLUSION
In summary, although much work has already been done for clinical indications other than radiation injury, in irradiated animal models, and a few first clinical applications for radiation injuries, there remain many questions as to the best cellular therapies and tissue sources to move forward for potential use in a radiation incident, as well as lingering concerns about their usability in a mass casualty scenario (e.g., storage, matching and administration). The provision of future funding in this scientific area should allow for some of these issues to be addressed, thus allowing for establishment of such therapies as optimum, life-saving approaches for use in the most heavily-irradiated patients. To that end, this conference provided data to inform future research objectives and focused funding opportunities to address these scientific gaps.
FIG. 1.
Mesenchymal stem cells (center) are thought to exert several protective effects in the body, including anti-inflammatory and immunomodulatory activities. They can be derived from many biological tissues, and can differentiate into a wide variety of cell types. For these reasons, their potential for clinical use is broad, and includes treatment of diseases ranging from immune dysfunction to tissue repair, as well as use in treating organ systems impacted by radiation exposure.
Acknowledgments
The authors thank the meeting participants (shown in Table 1) for providing their expertise and insight during both their presentations and in the discussion sessions. Thanks also to RNCP (David R. Cassatt, Merriline M. Satyamitra and Lanyn P. Taliaferro) and Office of Regulatory Affairs (Paul W. Price) colleagues for their critical review of the manuscript. Finally, the authors wish to acknowledge Sandra Barth at the IRSN, and Martha Jane Coury, within the NIAID Office of Global Research, for their invaluable assistance in planning and coordinating the meeting that led to this report.
Footnotes
Where pre-publication data are discussed, the investigator’s last name, to whom the information is attributed, is provided in parentheses.
References
- 1.Linard C, Tissedre F, Busson E, Holler V, Leclerc T, Strup-Perrot C, et al. Therapeutic potential of gingival fibroblasts for cutaneous radiation syndrome: comparison to bone marrow-mesenchymal stem cell grafts. Stem Cells Dev. 2015;24:1182–93. doi: 10.1089/scd.2014.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acharya MM, Christie LA, Hazel TG, Johe KK, Limoli CL. Transplantation of human fetal-derived neural stem cells improves cognitive function following cranial irradiation. Cell Transplant. 2014;23:1255–66. doi: 10.3727/096368913X670200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roy-Chowdhury N, Wang X, Guha C, Roy-Chowdhury J. Hepatocyte-like cells derived from induced pluripotent stem cells. Hepatol Int. 2016 doi: 10.1007/s12072-016-9757-y. [DOI] [PubMed] [Google Scholar]
- 4.Ende M. History of umbilical cord blood transplantation. Lancet. 1995;346:1161. doi: 10.1016/s0140-6736(95)91833-7. [DOI] [PubMed] [Google Scholar]
- 5.Gaberman E, Pinzur L, Levdansky L, Tsirlin M, Netzer N, Aberman Z, et al. Mitigation of lethal radiation syndrome in mice by intramuscular injection of 3D cultured adherent human placental stromal cells. PLoS One. 2013;8:e66549. doi: 10.1371/journal.pone.0066549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muramoto GG, Chen B, Cui X, Chao NJ, Chute JP. Vascular endothelial cells produce soluble factors that mediate the recovery of human hematopoietic stem cells after radiation injury. Biol Blood Marrow Transplant. 2006;12:530–40. doi: 10.1016/j.bbmt.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 7.Doan PL, Russell JL, Himburg HA, Helms K, Harris JR, Lucas J, et al. Tie2(+) bone marrow endothelial cells regulate hematopoietic stem cell regeneration following radiation injury. Stem Cells. 2013;31:327–37. doi: 10.1002/stem.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salter AB, Meadows SK, Muramoto GG, Himburg H, Doan P, Daher P, et al. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood. 2009;113:2104–7. doi: 10.1182/blood-2008-06-162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chute JP, Muramoto GG, Salter AB, Meadows SK, Rickman DW, Chen B, et al. Transplantation of vascular endothelial cells mediates the hematopoietic recovery and survival of lethally irradiated mice. Blood. 2007;109:2365–72. doi: 10.1182/blood-2006-05-022640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukumoto R. Mesenchymal stem cell therapy for acute radiation syndrome. Mil Med Res. 2016;3:17. doi: 10.1186/s40779-016-0086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton EB, Jr, Varney TR. Mesenchymal stem cell therapy for acute radiation syndrome: innovative medical approaches in military medicine. Mil Med Res. 2015;2:2. doi: 10.1186/s40779-014-0027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:57–66. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 13.Vangsness CT, Jr, Farr J, 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 2014;96:90–8. doi: 10.2106/JBJS.M.00058. [DOI] [PubMed] [Google Scholar]
- 14.Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143:1590–8. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaes B, Van’t Hof W, Deans R, Pinxteren J. Application of MultiStem((R)) Allogeneic Cells for Immunomodulatory Therapy: Clinical Progress and Pre-Clinical Challenges in Prophylaxis for Graft Versus Host Disease. Front Immunol. 2012;3:345. doi: 10.3389/fimmu.2012.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen BJ, Hou HS, Zhang HQ, Sui XW. Unrelated, HLA-mismatched multiple human umbilical cord blood transfusion in four cases with advanced solid tumors: initial studies. Blood Cells. 1994;20:285–92. [PubMed] [Google Scholar]
- 17.Azzam EI, Yang Z, Li M, Kim S, Kovalenko OA, Khorshidi M, et al. The effect of human cord blood therapy on the intestinal tract of lethally irradiated mice: possible use for mass casualties. Int J Radiat Biol. 2010;86:467–75. doi: 10.3109/09553000903567987. [DOI] [PubMed] [Google Scholar]
- 18.Czarneski J, Lin YC, Ende N, Ponzio NM, Raveche E. Effects of cord blood transfer on the hematopoietic recovery following sublethal irradiation in MRL lpr/lpr mice. Proc Soc Exp Biol Med. 1999;220:79–87. doi: 10.1046/j.1525-1373.1999.d01-13.x. [DOI] [PubMed] [Google Scholar]
- 19.Ende N, Lu S, Alcid MG, Chen R, Mack R. Pooled umbilical cord blood as a possible universal donor for marrow reconstitution and use in nuclear accidents. Life Sci. 2001;69:1531–9. doi: 10.1016/s0024-3205(01)01245-0. [DOI] [PubMed] [Google Scholar]
- 20.Ende N, Ponzio NM, Athwal RS, Ende M, Giuliani DC. Murine survival of lethal irradiation with the use of human umbilical cord blood. Life Sci. 1992;51:1249–53. doi: 10.1016/0024-3205(92)90013-f. [DOI] [PubMed] [Google Scholar]
- 21.Kovalenko OA, Azzam EI, Ende N. Human umbilical-cord-blood mononucleated cells enhance the survival of lethally irradiated mice: dosage and the window of time. Radiat Res. 2013;54:1010–4. doi: 10.1093/jrr/rrt062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ende N, Lu S, Ende M, Giuliani D, Ricafort RJ, Alcid MG, et al. Potential effectiveness of stored cord blood (non-frozen) for emergency use. J Emerg Med. 1996;14:673–7. doi: 10.1016/s0736-4679(96)00175-8. [DOI] [PubMed] [Google Scholar]
- 23.Scaradavou A, Isola L, Rubinstein P, Galperin Y, Najfeld V, Berlin D, et al. A murine model for human cord blood transplantation: near-term fetal and neonatal peripheral blood cells can achieve long-term bone marrow engraftment in sublethally irradiated adult recipients. Blood. 1997;89:1089–99. [PubMed] [Google Scholar]
- 24.Shim S, Lee SB, Lee JG, Jang WS, Lee SJ, Park S, et al. Mitigating effects of hUCB-MSCs on the hematopoietic syndrome resulting from total body irradiation. Exp Hematol. 2013;41:346–53. e2. doi: 10.1016/j.exphem.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Yi L, Zhang X, Gao L, Zhang C, Feng YM, et al. Cotransplantation of human umbilical cord blood-derived stromal cells enhances hematopoietic reconstitution and engraftment in irradiated BABL/c mice. Cancer Biol Ther. 2011;11:84–94. doi: 10.4161/cbt.11.1.13956. [DOI] [PubMed] [Google Scholar]
- 26.Nagayama H, Misawa K, Tanaka H, Ooi J, Iseki T, Tojo A, et al. Transient hematopoietic stem cell rescue using umbilical cord blood for a lethally irradiated nuclear accident victim. Bone Marrow Transplant. 2002;29:197–204. doi: 10.1038/sj.bmt.1703356. [DOI] [PubMed] [Google Scholar]
- 27.Lahiani A, Zahavi E, Netzer N, Ofir R, Pinzur L, Raveh S, et al. Human placental eXpanded (PLX) mesenchymal-like adherent stromal cells confer neuroprotection to nerve growth factor (NGF)-differentiated PC12 cells exposed to ischemia by secretion of IL-6 and VEGF. Biochimica et Biophysica Acta. 2015;1853:422–30. doi: 10.1016/j.bbamcr.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Cousin B, Andre M, Arnaud E, Penicaud L, Casteilla L. Reconstitution of lethally irradiated mice by cells isolated from adipose tissue. Biochem Biophysical Res Commun. 2003;301:1016–22. doi: 10.1016/s0006-291x(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 29.Kharfan-Dabaja MA, Kumar A, Hamadani M, Stilgenbauer S, Ghia P, Anasetti C, et al. Clinical practice recommendations for use of allogeneic hematopoietic cell transplantation in chronic lymphocytic leukemia on behalf of the guidelines committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2016 doi: 10.1016/j.bbmt.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rashidi A, Ebadi M, Cashen AF. Allogeneic hematopoietic stem cell transplantation in Hodgkin lymphoma: a systematic review and meta-analysis. Bone Marrow Transplant. 2016;51:521–8. doi: 10.1038/bmt.2015.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martino M, Lemoli RM, Girmenia C, Castagna L, Bruno B, Cavallo F, et al. Italian consensus conference for the outpatient autologous stem cell transplantation management in multiple myeloma. Bone Marrow Transplant. 2016;51:1032–40. doi: 10.1038/bmt.2016.79. [DOI] [PubMed] [Google Scholar]
- 32.Bolanos-Meade J, Brodsky RA. Blood and marrow transplantation for sickle cell disease: is less more? Blood Rev. 2014;28:243–8. doi: 10.1016/j.blre.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ringden O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lonnies H, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–7. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 34.Fang B, Song YP, Liao LM, Han Q, Zhao RC. Treatment of severe therapy-resistant acute graft-versus-host disease with human adipose tissue-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;38:389–90. doi: 10.1038/sj.bmt.1705457. [DOI] [PubMed] [Google Scholar]
- 35.Honmou O, Onodera R, Sasaki M, Waxman SG, Kocsis JD. Mesenchymal stem cells: therapeutic outlook for stroke. Trends Mol Med. 2012;18:292–7. doi: 10.1016/j.molmed.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Dryden GW. Overview of stem cell therapy for Crohn’s disease. Expert Opin Biol Ther. 2009;9:841–7. doi: 10.1517/14712590902956615. [DOI] [PubMed] [Google Scholar]
- 37.Inamdar AC, Inamdar AA. Mesenchymal stem cell therapy in lung disorders: pathogenesis of lung diseases and mechanism of action of mesenchymal stem cell. Exp Lung Res. 2013;39:315–27. doi: 10.3109/01902148.2013.816803. [DOI] [PubMed] [Google Scholar]
- 38.Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H, et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467–75. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 39.Sheng CC, Zhou L, Hao J. Current stem cell delivery methods for myocardial repair. BioMed Res Int. 2013;2013:547902. doi: 10.1155/2013/547902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jean Kunlin Dardik H. Correspondence and commentary regarding distal fistulas for lower limb ischemia. J Vasc Surg. 2016 doi: 10.1016/j.jvs.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 41.Maufus M, Antoinette Sevestre-Pietri M, Sessa C, Pignon B, Egelhofer H, Dupas S, et al. Critical limb ischaemia and the response to bone marrow-derived cell therapy according to tcPO2 measurement. Vasa. 2016:1–6. doi: 10.1024/0301-1526/a000590. [DOI] [PubMed] [Google Scholar]
- 42.Monteiro Carvalho Mori da Cunha MG, Zia S, Oliveira Arcolino F, Carlon MS, Beckmann DV, Pippi NL, et al. Amniotic fluid derived stem cells with a renal progenitor phenotype inhibit interstitial fibrosis in renal ischemia and reperfusion injury in rats. PLoS One. 2015;10:e0136145. doi: 10.1371/journal.pone.0136145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou L, Xu L, Shen J, Song Q, Wu R, Ge Y, et al. Preischemic administration of nonexpanded adipose stromal vascular fraction attenuates acute renal ischemia/reperfusion injury and fibrosis. Stem Cells Transl Med. 2016;5:1277–88. doi: 10.5966/sctm.2015-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Office of Counterterrorism and Emerging Threats, Food and Drug Administration. Emergency use authorization of medical products and related authorities. 2017. [Google Scholar]
- 45.Ancans J. Cell therapy medicinal product regulatory framework in Europe and its application for MSC-based therapy development. Front Immunol. 2012;3:253. doi: 10.3389/fimmu.2012.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferry N. European regulation for therapeutic use of stem cells. Biomed Mater Eng. 2017;28:S3–S7. doi: 10.3233/BME-171619. [DOI] [PubMed] [Google Scholar]
- 47.Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) Guidance for Industry. 2015. Product Development Under the Animal Rule. [Google Scholar]
- 48.Food and Drug Administration, Center for Biologics Evaluation and Research (CBER) Guidance for industry: Preclinical assessment of investigational cellular and gene therapy products. 2013. [Google Scholar]
- 49.Food and Drug Administration (FDA), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH), Office of Combination Products (OCP) Homologous Use of Human Cells, Tissues, and Cellular and Tissue-Based Products: Draft Guidance for Industry and FDA Staff. 2015. [Google Scholar]
- 50.Food and Drug Administration (FDA), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH), Office of Combination Products in the Office of the Commissioner (OCP) Draft Guidance for Industry. 2014. Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) from Adipose Tissue: Regulatory Considerations. [Google Scholar]
- 51.Guidance for industry: Expedited programs for serious conditions - drugs and biologics. 2014.
- 52.Erben RG, Silva-Lima B, Reischl I, Steinhoff G, Tiedemann G, Dalemans W, et al. White paper on how to go forward with cell-based advanced therapies in Europe. Tissue Eng Part A. 2014;20:2549–54. doi: 10.1089/ten.tea.2013.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guillaume-Jugnot P, Daumas A, Magalon J, Jouve E, Nguyen PS, Truillet R, et al. Autologous adipose-derived stromal vascular fraction in patients with systemic sclerosis: 12-month follow-up. Rheumatology (Oxford) 2016;55:301–6. doi: 10.1093/rheumatology/kev323. [DOI] [PubMed] [Google Scholar]
- 54.Guillaume-Jugnot P, Daumas A, Magalon J, Sautereau N, Veran J, Magalon G, et al. State of the art. Autologous fat graft and adipose tissue-derived stromal vascular fraction injection for hand therapy in systemic sclerosis patients. Curr Res Transl Med. 2016;64:35–42. doi: 10.1016/j.retram.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 55.Granel B, Daumas A, Jouve E, Harle JR, Nguyen PS, Chabannon C, et al. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: an open-label phase I trial. Ann Rheum Dis. 2015;74:2175–82. doi: 10.1136/annrheumdis-2014-205681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daumas A, Magalon J, Jouve E, Truillet R, Casanova D, Giraudo L, et al. Long-term follow-up after autologous adipose-derived stromal vascular fraction injection into fingers in systemic sclerosis patients. Curr Res Transl Med. 2017;65:40–3. doi: 10.1016/j.retram.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 57.Foubert P, Gonzalez AD, Teodosescu S, Berard F, Doyle-Eisele M, Yekkala K, et al. Adipose-derived regenerative cell therapy for burn wound healing: A comparison of two delivery methods. Adv Wound Care (New Rochelle) 2016;5:288–98. doi: 10.1089/wound.2015.0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foubert P, Doyle-Eisele M, Gonzalez A, Berard F, Weber W, Zafra D, et al. Development of a combined radiation and full thickness burn injury minipig model to study the effects of uncultured adipose-derived regenerative cell therapy in wound healing. Int J Radiat Biol. 2016:1–35. doi: 10.1080/09553002.2017.1242814. [DOI] [PubMed] [Google Scholar]
- 59.Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell. 2014;14:141–5. doi: 10.1016/j.stem.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 60.Bellayr IH, Marklein RA, Lo Surdo JL, Bauer SR, Puri RK. Identification of predictive gene markers for multipotent stromal cell proliferation. Stem Cells Dev. 2016;25:861–73. doi: 10.1089/scd.2015.0374. [DOI] [PubMed] [Google Scholar]
- 61.Bellayr IH, Catalano JG, Lababidi S, Yang AX, Lo Surdo JL, Bauer SR, et al. Gene markers of cellular aging in human multipotent stromal cells in culture. Stem Cell Res Ther. 2014;5:59. doi: 10.1186/scrt448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lynch PJ, Thompson EE, McGinnis K, Rovira Gonzalez YI, Lo Surdo J, Bauer SR, et al. Chromatin changes at the PPAR-gamma2 promoter during bone marrow-derived multipotent stromal cell culture correlate with loss of gene activation potential. Stem Cells. 2015;33:2169–81. doi: 10.1002/stem.1967. [DOI] [PubMed] [Google Scholar]
- 63.Mindaye ST, Ra M, Lo Surdo J, Bauer SR, Alterman MA. Improved proteomic profiling of the cell surface of culture-expanded human bone marrow multipotent stromal cells. J Proteomics. 2013;78:1–14. doi: 10.1016/j.jprot.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 64.Lo Surdo J, Bauer SR. Quantitative approaches to detect donor and passage differences in adipogenic potential and clonogenicity in human bone marrow-derived mesenchymal stem cells. Tissue Eng Part C, Methods. 2012;18:877–89. doi: 10.1089/ten.tec.2011.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nazarov C, Lo Surdo J, Bauer SR, Wei CH. Assessment of immunosuppressive activity of human mesenchymal stem cells using murine antigen specific CD4 and CD8 T cells in vitro. Stem Cell Res Ther. 2013;4:128. doi: 10.1186/scrt339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–86. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 67.von Bonin M, Stolzel F, Goedecke A, Richter K, Wuschek N, Holig K, et al. Treatment of refractory acute GVHD with third-party MSC expanded in platelet lysate-containing medium. Bone Marrow Transplant. 2009;43:245–51. doi: 10.1038/bmt.2008.316. [DOI] [PubMed] [Google Scholar]
- 68.Galipeau J. The mesenchymal stromal cells dilemma-does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15:2–8. doi: 10.1016/j.jcyt.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 69.Jellema RK, Ophelders DR, Zwanenburg A, Nikiforou M, Delhaas T, Andriessen P, et al. Multipotent adult progenitor cells for hypoxic-ischemic injury in the preterm brain. J Neuroinflammation. 2015;12:241. doi: 10.1186/s12974-015-0459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L Therapy MSCCotISfC. Immunological characterization of multipotent mesenchymal stromal cells-The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013;15:1054–61. doi: 10.1016/j.jcyt.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 71.Francois M, Copland IB, Yuan S, Romieu-Mourez R, Waller EK, Galipeau J. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-gamma licensing. Cytotherapy. 2012;14:147–52. doi: 10.3109/14653249.2011.623691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chinnadurai R, Garcia MA, Sakurai Y, Lam WA, Kirk AD, Galipeau J, et al. Actin cytoskeletal disruption following cryopreservation alters the biodistribution of human mesenchymal stromal cells in vivo. Stem Cell Reports. 2014;3:60–72. doi: 10.1016/j.stemcr.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moll G, Alm JJ, Davies LC, von Bahr L, Heldring N, Stenbeck-Funke L, et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014;32:2430–42. doi: 10.1002/stem.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Otsuru S, Hofmann TJ, Raman P, Olson TS, Guess AJ, Dominici M, et al. Genomic and functional comparison of mesenchymal stromal cells prepared using two isolation methods. Cytotherapy. 2015;17:262–70. doi: 10.1016/j.jcyt.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 75.Li M, Zhao Y, Hao H, Dong L, Liu J, Han W, et al. Umbilical cord-derived mesenchymal stromal cell-conditioned medium exerts in vitro antiaging effects in human fibroblasts. Cytotherapy. 2017;19:371–83. doi: 10.1016/j.jcyt.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 76.Sotiropoulou PA, Candi A, Mascre G, De Clercq S, Youssef KK, Lapouge G, et al. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat Cell Biol. 2010;12:572–82. doi: 10.1038/ncb2059. [DOI] [PubMed] [Google Scholar]
- 77.Revenco T, Lapouge G, Moers V, Brohee S, Sotiropoulou PA. Low dose radiation causes skin cancer in mice and has a differential effect on distinct epidermal stem cells. Stem Cells. 2017 doi: 10.1002/stem.2571. [DOI] [PubMed] [Google Scholar]
- 78.Horton JA, Hudak KE, Chung EJ, White AO, Scroggins BT, Burkeen JF, et al. Mesenchymal stem cells inhibit cutaneous radiation-induced fibrosis by suppressing chronic inflammation. Stem Cells. 2013;31:2231–41. doi: 10.1002/stem.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Inomata K, Aoto T, Binh NT, Okamoto N, Tanimura S, Wakayama T, et al. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell. 2009;137:1088–99. doi: 10.1016/j.cell.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 80.Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 81.Rodriguez-Menocal L, Shareef S, Salgado M, Shabbir A, Van Badiavas E. Role of whole bone marrow, whole bone marrow cultured cells, and mesenchymal stem cells in chronic wound healing. Stem Cell Res Ther. 2015;6:24. doi: 10.1186/s13287-015-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Agay D, Scherthan H, Forcheron F, Grenier N, Herodin F, Meineke V, et al. Multipotent mesenchymal stem cell grafting to treat cutaneous radiation syndrome: development of a new minipig model. Exp Hematol. 2010;38:945–56. doi: 10.1016/j.exphem.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 83.Rezvani M. Stem cell derived therapy for cutaneous radiation exposure. Hautarzt. 2013;64:910–6. doi: 10.1007/s00105-013-2629-7. [DOI] [PubMed] [Google Scholar]
- 84.Riccobono D, Agay D, Francois S, Scherthan H, Drouet M, Forcheron F. Contribution of INTRAMUSCULAR Autologous Adipose Tissue-Derived Stem Cell Injections to Treat Cutaneous Radiation Syndrome: Preliminary Results. Health Phys. 2016;111:117–26. doi: 10.1097/HP.0000000000000515. [DOI] [PubMed] [Google Scholar]
- 85.Riccobono D, Forcheron F, Agay D, Scherthan H, Meineke V, Drouet M. Transient gene therapy to treat cutaneous radiation syndrome: development in a minipig model. Health Phys. 2014;106:713–9. doi: 10.1097/HP.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 86.Bey E, Prat M, Duhamel P, Benderitter M, Brachet M, Trompier F, et al. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen. 2010;18:50–8. doi: 10.1111/j.1524-475X.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- 87.Lataillade JJ, Doucet C, Bey E, Carsin H, Huet C, Clairand I, et al. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen Medicine. 2007;2:785–94. doi: 10.2217/17460751.2.5.785. [DOI] [PubMed] [Google Scholar]
- 88.Gottlober P, Bezold G, Weber L, Gourmelon P, Cosset JM, Bahren W, et al. The radiation accident in Georgia: clinical appearance and diagnosis of cutaneous radiation syndrome. J Am Acad Dermatol. 2000;42:453–8. doi: 10.1016/s0190-9622(00)90218-4. [DOI] [PubMed] [Google Scholar]
- 89.Barriga LE, Zaharia M, Pinillos L, Moscol A, Heredia A, Sarria G, et al. Long-term follow-up of radiation accident patients in Peru: review of two cases. Radiat Prot Dosimetry. 2012;151:652–5. doi: 10.1093/rpd/ncs175. [DOI] [PubMed] [Google Scholar]
- 90.The radiological accident in Nueva Aldea. IAEA; Vienna, Austria: 2009. [Google Scholar]
- 91.Ebrahimian TG, Pouzoulet F, Squiban C, Buard V, Andre M, Cousin B, et al. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler Thromb Vasc Biol. 2009;29:503–10. doi: 10.1161/ATVBAHA.108.178962. [DOI] [PubMed] [Google Scholar]
- 92.Xie MW, Gorodetsky R, Micewicz ED, Mackenzie NC, Gaberman E, Levdansky L, et al. Marrow-derived stromal cell delivery on fibrin microbeads can correct radiation-induced wound-healing deficits. J Invest Dermatol. 2013;133:553–61. doi: 10.1038/jid.2012.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Latil M, Rocheteau P, Chatre L, Sanulli S, Memet S, Ricchetti M, et al. Skeletal muscle stem cells adopt a dormant cell state post mortem and retain regenerative capacity. Nat Commun. 2012;3:903. doi: 10.1038/ncomms1890. [DOI] [PubMed] [Google Scholar]
- 94.Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, Tajbakhsh S. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell. 2012;148:112–25. doi: 10.1016/j.cell.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 95.Vahidi Ferdousi L, Rocheteau P, Chayot R, Montagne B, Chaker Z, Flamant P, et al. More efficient repair of DNA double-strand breaks in skeletal muscle stem cells compared to their committed progeny. Stem Cell Res. 2014;13:492–507. doi: 10.1016/j.scr.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 96.Andrews GA. Criticality accidents in Vinca, Yugoslavia, and Oak Ridge, Tennessee. Comparison of radiation injuries and results of therapy. JAMA. 1962;179:191–7. doi: 10.1001/jama.1962.03050030005002. [DOI] [PubMed] [Google Scholar]
- 97.Baranov A, Gale RP, Guskova A, Piatkin E, Selidovkin G, Muravyova L, et al. Bone marrow transplantation after the Chernobyl nuclear accident. N Engl J Med. 1989;321:205–12. doi: 10.1056/NEJM198907273210401. [DOI] [PubMed] [Google Scholar]
- 98.Butturini A, De Souza PC, Gale RP, Cordiero JM, Lopes DM, Neto C, et al. Use of recombinant granulocyte-macrophage colony stimulating factor in the Brazil radiation accident. Lancet. 1988;2:471–5. doi: 10.1016/s0140-6736(88)90121-3. [DOI] [PubMed] [Google Scholar]
- 99.Densow D, Kindler H, Baranov AE, Tibken B, Hofer EP, Fliedner TM. Criteria for the selection of radiation accident victims for stem cell transplantation. Stem Cells. 1997;15(Suppl 2):287–97. doi: 10.1002/stem.5530150738. [DOI] [PubMed] [Google Scholar]
- 100.Dainiak N, Gent RN, Carr Z, Schneider R, Bader J, Buglova E, et al. First global consensus for evidence-based management of the hematopoietic syndrome resulting from exposure to ionizing radiation. Disaster Med Public Health Prep. 2011;5:202–12. doi: 10.1001/dmp.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hirama T, Tanosaki S, Kandatsu S, Kuroiwa N, Kamada T, Tsuji H, et al. Initial medical management of patients severely irradiated in the Tokai-mura criticality accident. Br J Radiol. 2003;76:246–53. doi: 10.1259/bjr/82373369. [DOI] [PubMed] [Google Scholar]
- 102.Li B, Bailey AS, Jiang S, Liu B, Goldman DC, Fleming WH. Endothelial cells mediate the regeneration of hematopoietic stem cells. Stem Cell Res. 2010;4:17–24. doi: 10.1016/j.scr.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rafii S, Ginsberg M, Scandura J, Butler JM, Ding BS. Transplantation of endothelial cells to mitigate acute and chronic radiation injury to vital organs. Radiat Res. 2016;186:196–202. doi: 10.1667/RR14461.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310–5. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Farese AM, Cohen MV, Katz BP, Smith CP, Gibbs A, Cohen DM, et al. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat Res. 2013;179:89–100. doi: 10.1667/RR3049.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bertho JM, Prat M, Frick J, Demarquay C, Gaugler MH, Dudoignon N, et al. Application of autologous hematopoietic cell therapy to a nonhuman primate model of heterogeneous high-dose irradiation. Radiat Res. 2005;163:557–70. doi: 10.1667/rr3352. [DOI] [PubMed] [Google Scholar]
- 107.Gourmelon P, Benderitter M, Bertho JM, Huet C, Gorin NC, De Revel P. European consensus on the medical management of acute radiation syndrome and analysis of the radiation accidents in Belgium and Senegal. Health Phys. 2010;98:825–32. doi: 10.1097/HP.0b013e3181ce64d4. [DOI] [PubMed] [Google Scholar]
- 108.Gorin NC, Fliedner TM, Gourmelon P, Ganser A, Meineke V, Sirohi B, et al. Consensus conference on European preparedness for haematological and other medical management of mass radiation accidents. Ann Hematol. 2006;85:671–9. doi: 10.1007/s00277-006-0153-x. [DOI] [PubMed] [Google Scholar]
- 109.Fliedner TM, Chao NJ, Bader JL, Boettger A, Case C, Jr, Chute J, et al. Stem cells, multiorgan failure in radiation emergency medical preparedness: a U.S./European Consultation Workshop. Stem Cells. 2009;27:1205–11. doi: 10.1002/stem.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bertho JM, Roy L, Souidi M, Benderitter M, Bey E, Racine R, et al. Initial evaluation and follow-up of acute radiation syndrome in two patients from the Dakar accident. Biomarkers. 2009;14:94–102. doi: 10.1080/13547500902773904. [DOI] [PubMed] [Google Scholar]
- 111.The radiological accident in Soreq. International Atomic Energy Agency; Vienna, Austria: 1993. [Google Scholar]
- 112.The radiological accident at the irradiation facility in Nesvizh. IAEA; Vienna, Austria: 1996. [Google Scholar]
- 113.Igaki H, Nakagawa K, Uozaki H, Akahane M, Hosoi Y, Fukayama M, et al. Pathological changes in the gastrointestinal tract of a heavily radiation-exposed worker at the Tokai-mura criticality accident. Radiat Res. 2008;49:55–62. doi: 10.1269/jrr.07058. [DOI] [PubMed] [Google Scholar]
- 114.Friesecke I, Beyrer K, Wedel R, Reimers K, Fliedner TM. SEARCH: a system for evaluation and archiving of radiation accidents based on case histories. Radiat Enviro Biophys. 2000;39:213–7. doi: 10.1007/s004110000056. [DOI] [PubMed] [Google Scholar]
- 115.Fliedner TM, Powles R, Sirohi B, Niederwieser D European Group for B, Marrow Transplantation Nuclear Accident C. Radiologic and nuclear events: the METREPOL severity of effect grading system. Blood. 2008;111:5757–8. doi: 10.1182/blood-2008-04-150243. author reply 8–9. [DOI] [PubMed] [Google Scholar]
- 116.Singh VK, Newman VL, Romaine PL, Wise SY, Seed TM. Radiation countermeasure agents: an update (2011–2014) Expert Opin Ther Pat. 2014;24:1229–55. doi: 10.1517/13543776.2014.964684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, Guha C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One. 2011;6:e24072. doi: 10.1371/journal.pone.0024072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chang YH, Lin LM, Lou CW, Chou CK, Ch’ang HJ. Bone marrow transplantation rescues intestinal mucosa after whole body radiation via paracrine mechanisms. Radiother Oncol. 2012;105:371–7. doi: 10.1016/j.radonc.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 119.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–7. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 120.Semont A, Mouiseddine M, Francois A, Demarquay C, Mathieu N, Chapel A, et al. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2010;17:952–61. doi: 10.1038/cdd.2009.187. [DOI] [PubMed] [Google Scholar]
- 121.Singh VK, Christensen J, Fatanmi OO, Gille D, Ducey EJ, Wise SY, et al. Myeloid progenitors: a radiation countermeasure that is effective when initiated days after irradiation. Radiat Res. 2012;177:781–91. doi: 10.1667/rr2894.1. [DOI] [PubMed] [Google Scholar]
- 122.Peiffert D, Simon JM, Eschwege F. Epinal radiotherapy accident: passed, present, future. Cancer Radiother. 2007;11:309–12. doi: 10.1016/j.canrad.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 123.Voswinkel J, Francois S, Simon JM, Benderitter M, Gorin NC, Mohty M, et al. Use of mesenchymal stem cells (MSC) in chronic inflammatory fistulizing and fibrotic diseases: a comprehensive review. Clin Rev Allergy Immunol. 2013;45:180–92. doi: 10.1007/s12016-012-8347-6. [DOI] [PubMed] [Google Scholar]
- 124.Yamada M, Wong FL, Fujiwara S, Akahoshi M, Suzuki G. Noncancer disease incidence in atomic bomb survivors, 1958–1998. Radiat Res. 2004;161:622–32. doi: 10.1667/rr3183. [DOI] [PubMed] [Google Scholar]
- 125.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: solid cancer and noncancer disease mortality: 1950–1997. 2003. Radiat Res. 2012;178:AV146–72. doi: 10.1667/rrav12.1. [DOI] [PubMed] [Google Scholar]
- 126.Yablokov AV. 5. Nonmalignant diseases after the Chernobyl catastrophe. Ann N Y Acad Sci. 2009;1181:58–160. doi: 10.1111/j.1749-6632.2009.04826.x. [DOI] [PubMed] [Google Scholar]
- 127.Leeman KT, Fillmore CM, Kim CF. Lung stem and progenitor cells in tissue homeostasis and disease. Curr Top Dev Biol. 2014;107:207–33. doi: 10.1016/B978-0-12-416022-4.00008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Theise ND, Henegariu O, Grove J, Jagirdar J, Kao PN, Crawford JM, et al. Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol. 2002;30:1333–8. doi: 10.1016/s0301-472x(02)00931-1. [DOI] [PubMed] [Google Scholar]
- 129.Fischer UM, Harting MT, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, et al. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18:683–92. doi: 10.1089/scd.2008.0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang YY, Li XZ, Wang LB. Therapeutic implications of mesenchymal stem cells in acute lung injury/acute respiratory distress syndrome. Stem Cell Res Ther. 2013;4:45. doi: 10.1186/scrt193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Krause DS. Bone marrow-derived lung epithelial cells. Proc Am Thorac Soc. 2008;5:699–702. doi: 10.1513/pats.200803-031AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Iwata M, Madtes DK, Abrams K, Lamm WJ, Glenny RW, Nash RA, et al. Late infusion of cloned marrow fibroblasts stimulates endogenous recovery from radiation-induced lung injury. PLoS One. 2013;8:e57179. doi: 10.1371/journal.pone.0057179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. Clin Oncol. 2006;24:1305–9. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 134.Acharya MM, Martirosian V, Christie LA, Limoli CL. Long-term cognitive effects of human stem cell transplantation in the irradiated brain. Int J Radiat Biol. 2014;90:816–20. doi: 10.3109/09553002.2014.927934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Baulch JE, Acharya MM, Allen BD, Ru N, Chmielewski NN, Martirosian V, et al. Cranial grafting of stem cell-derived microvesicles improves cognition and reduces neuropathology in the irradiated brain. Proc Natl Acad Sci U S A. 2016;113:4836–41. doi: 10.1073/pnas.1521668113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pringle S, Maimets M, van der Zwaag M, Stokman MA, van Gosliga D, Zwart E, et al. Human salivary gland stem cells functionally restore radiation damaged salivary glands. Stem Cells. 2016;34:640–52. doi: 10.1002/stem.2278. [DOI] [PubMed] [Google Scholar]
- 137.Pringle S, Van Os R, Coppes RP. Concise review: Adult salivary gland stem cells and a potential therapy for xerostomia. Stem Cells. 2013;31:613–9. doi: 10.1002/stem.1327. [DOI] [PubMed] [Google Scholar]
- 138.van Luijk P, Pringle S, Deasy JO, Moiseenko VV, Faber H, Hovan A, et al. Sparing the region of the salivary gland containing stem cells preserves saliva production after radiotherapy for head and neck cancer. Science TransC Med. 2015;7:305ra147. doi: 10.1126/scitranslmed.aac4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lombaert IM, Wierenga PK, Kok T, Kampinga HH, deHaan G, Coppes RP. Mobilization of bone marrow stem cells by granulocyte colony-stimulating factor ameliorates radiation-induced damage to salivary glands. Clin Cancer Res. 2006;12:1804–12. doi: 10.1158/1078-0432.CCR-05-2381. [DOI] [PubMed] [Google Scholar]
- 140.Lombaert IM, Brunsting JF, Wierenga PK, Kampinga HH, de Haan G, Coppes RP. Cytokine treatment improves parenchymal and vascular damage of salivary glands after irradiation. Clin Cancer Res. 2008;14:7741–50. doi: 10.1158/1078-0432.CCR-08-1449. [DOI] [PubMed] [Google Scholar]
- 141.Nanduri LS, Baanstra M, Faber H, Rocchi C, Zwart E, de Haan G, et al. Purification and ex vivo expansion of fully functional salivary gland stem cells. Stem Cell Reports. 2014;3:957–64. doi: 10.1016/j.stemcr.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maimets M, Rocchi C, Bron R, Pringle S, Kuipers J, Giepmans BN, et al. Long-term in vitro expansion of salivary gland stem cells driven by Wnt signals. Stem Cell Reports. 2016;6:150–62. doi: 10.1016/j.stemcr.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Abbo O, Taurand M, Monsarrat P, Raymond I, Arnaud E, De Barros S, et al. Comparison between pediatric and adult adipose mesenchymal stromal cells. Cytotherapy. 2017;19:395–407. doi: 10.1016/j.jcyt.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 144.Maimets M, Bron R, de Haan G, van Os R, Coppes RP. Similar ex vivo expansion and post-irradiation regenerative potential of juvenile and aged salivary gland stem cells. Radiother Oncol. 2015;116:443–8. doi: 10.1016/j.radonc.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 145.Schatteman GC, Ma N. Old bone marrow cells inhibit skin wound vascularization. Stem Cells. 2006;24:717–21. doi: 10.1634/stemcells.2005-0214. [DOI] [PubMed] [Google Scholar]
- 146.Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102:1319–30. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tepper OM, Carr J, Allen RJ, Jr, Chang CC, Lin CD, Tanaka R, et al. Decreased circulating progenitor cell number and failed mechanisms of stromal cell-derived factor-1alpha mediated bone marrow mobilization impair diabetic tissue repair. Diabetes. 2010;59:1974–83. doi: 10.2337/db09-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee HC, An SG, Lee HW, Park JS, Cha KS, Hong TJ, et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: a pilot study. Circ J. 2012;76:1750–60. doi: 10.1253/circj.cj-11-1135. [DOI] [PubMed] [Google Scholar]
- 149.Gahrton G, Iacobelli S, Apperley J, Bandini G, Bjorkstrand B, Blade J, et al. The impact of donor gender on outcome of allogeneic hematopoietic stem cell transplantation for multiple myeloma: reduced relapse risk in female to male transplants. Bone Marrow Transplant. 2005;35:609–17. doi: 10.1038/sj.bmt.1704861. [DOI] [PubMed] [Google Scholar]
- 150.Hovden AO, Appel S. The first dendritic cell-based therapeutic cancer vaccine is approved by the FDA. Scand J Immunol. 2010;72:554. doi: 10.1111/j.1365-3083.2010.02464.x. [DOI] [PubMed] [Google Scholar]
- 151.Tran SD, Liu Y, Xia D, Maria OM, Khalili S, Wang RW, et al. Paracrine effects of bone marrow soup restore organ function, regeneration, and repair in salivary glands damaged by irradiation. PLoS One. 2013;8:e61632. doi: 10.1371/journal.pone.0061632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stultz BG, McGinnis K, Thompson EE, Lo Surdo JL, Bauer SR, Hursh DA. Chromosomal stability of mesenchymal stromal cells during in vitro culture. Cytotherapy. 2016;18:336–43. doi: 10.1016/j.jcyt.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.DiCarlo AL, Maher C, Hick JL, Hanfling D, Dainiak N, Chao N, et al. Radiation injury after a nuclear detonation: medical consequences and the need for scarce resources allocation. Disaster Med Public Health Prep. 2011;5(Suppl 1):S32–44. doi: 10.1001/dmp.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Poglio S, Galvani S, Bour S, Andre M, Prunet-Marcassus B, Penicaud L, et al. Adipose tissue sensitivity to radiation exposure. Am J Pathol. 2009;174:44–53. doi: 10.2353/ajpath.2009.080505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nagle PW, Hosper NA, Ploeg EM, van Goethem MJ, Brandenburg S, Langendijk JA, et al. The in vitro response of tissue stem cells to irradiation with different linear energy transfers. Int J Radiat Oncol Biol Phys. 2016;95:103–11. doi: 10.1016/j.ijrobp.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 156.Shin HS, An HY, Choi JS, Kim HJ, Lim JY. Organotypic spheroid culture to mimic radiation-induced salivary hypofunction. J Dent Res. 2016 doi: 10.1177/0022034516685036. 22034516685036. [DOI] [PubMed] [Google Scholar]
- 157.Ross JR, Case C, Confer D, Weisdorf DJ, Weinstock D, Krawisz R, et al. Radiation injury treatment network (RITN): healthcare professionals preparing for a mass casualty radiological or nuclear incident. Int J Radiat Biol. 2011;87:748–53. doi: 10.3109/09553002.2011.556176. [DOI] [PMC free article] [PubMed] [Google Scholar]