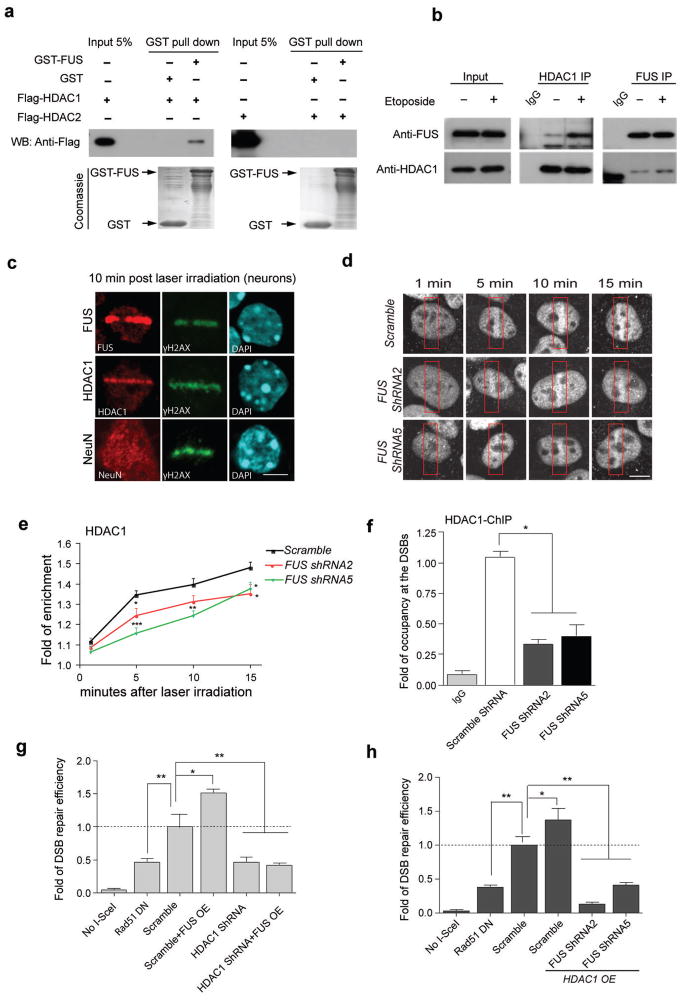
Figure 3. FUS directly interacts with HDAC1, and both proteins are necessary for successful DNA repair.
(a) Purified GST-FUS protein was incubated with recombinant Flag-HDAC1 or Flag-HDAC2, precipitated with anti-GST beads, and blotted with anti-Flag antibody. A duplicate SDS-PAGE gel was stained with Coomassie blue as input controls. (b) Immunoprecipitation and blotting with antibodies against HDAC1 and FUS in cultured primary neurons with 1 h etoposide or vehicle treatment. Full-length blots are presented in Supplemental Fig. 10. (c) Immunoreactivity for FUS, HDAC1, NeuN and γH2AX in micro-irradiated primary cortical neurons. Scale bar, 4μm. (d, e) HDAC1 immunolabeling of U2OS cells that were infected with indicated shRNA-expressing lentiviruses, and were subjected to laser micro-irradiation. Red boxes indicate damaged area. Cells were fixed and stained for endogenous HDAC1 at indicated time following irradiation. Scale bar = 4μm. Fold enrichment of HDAC1 signal intensity at the lesioned area compared to the whole nucleus was quantified as a function of time following irradiation (mean ± SEM, n = 6–11, *P<0.05, **P<0.01, ***P<0.001, unpaired t-test). (f) The U2OS-GFP cell line, transduced with indicated lentivirus, was subjected to ChIP followed by qPCR to evaluate the occupancy of HDAC1 to the DSBs created by I-SceI (mean ± SEM, *P<0.05, one-way ANOVA). (g,h) DSB repair efficiency was evaluated in U2OS-GFP cells by co-transfecting the indicated constructs together with I-SceI. Percentage of GFP+ cells was analyzed by FACS and fold changes were normalized to cells transduced with lentivirus carrying scrambled shRNA (mean ± SEM, *P<0.05, **P<0.01, unpaired t-test).