Abstract
Renal cell carcinoma is one of the most common malignancy in adults, its prognosis is poor in an advanced stage and early detection is difficult due to the lack of molecular biomarkers. The identification of novel biomarkers for RCC is an urgent and meaningful project. Long non-coding RNA (lncRNA) is transcribed from genomic regions with a minimum length of 200 bases and limited protein-coding potential. Recently, lncRNAs have been greatly studied in a variety of cancer types. They participate in a wide variety of biological processes including cancer biology. In this review, we provide a new insight of the profiling of lncRNAs in RCC and their roles in renal carcinogenesis, with an emphasize on their potential in diagnosis, prognosis and potential roles in RCC therapy.
Keywords: renal cell carcinoma, lncRNA, diagnosis, prognosis, therapy
INTRODUCTION
Renal cell carcinoma (RCC) is one of the most common urinary tract malignancies in adults. In 2016, 62,700 newly identified cases and 14240 deaths from kidney and renal pelvis, cancer was estimated to occur in the United States [1]. With mortality at about 3% of all cases and the rate continues to remain very high [1]. In China, an estimated of 66.8 ‰ new cases and 23.4 ‰ deaths from renal cancer occurred in 2015 [2]. The most prevalent RCC histological subtype is clear cell RCC (ccRCC), which accounts for 70% of RCC, followed by papillary RCC (pRCC) and chromophobe RCC (chRCC) (with a prevalence of 10 and 5%, respectively) [3]. In 2016, world health organization (WHO) modified the classification of RCC and added several newly recognized renal tumors [4].
The tumorigenesis of RCC is extremely complex, not only entailing genetic changes but also including dysregulation of epigenetic pathways [5]. Many well-known key signal transduction pathways such as VHL/HIF, PI3K/Akt/mTOR, Raf/MAPK/ERK Jak/Stat, and Wnt-β-catenin pathway have been demonstrated to be involved in the pathogenesis and development of RCC [6–10]. Although great insight into the epigenetics of RCC has been made, for instance, DNA methylation [11], histone modification [12], as well as noncoding RNA [13]. But the underlying epigenetic mechanisms still need to be further studied. Currently, there are no specific and effective molecular biomarkers for RCC. Thus, the identification of novel biomarkers for RCC is extremely urgent and maybe further serve as therapeutic targets for the treatment.
It is estimated that 80% of the human genome is transcribed. But only 1-2% of the whole genome is protein-encoded. Remaining non-coding portions of the genome transcription products are non-coding RNAs, they are different in biogenesis, properties and functions [14, 15]. There are many famous non-cording RNAs, microRNAs (miRNAs), small interfering RNAs (siRNAs), transfer RNAs (tRNAs), ribosomal RNAs (rRNAs), small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), all of which play critical roles in mammalian cells [16]. Most of them are transcribed by RNA polymerase II; some are transcribed by RNA polymerase III [16]. They participate in the epigenetic regulation of other molecules, binding to DNAs, proteins and/or other RNAs [17, 18]. Recently, a newly discovered type of long non-coding RNA molecules (lncRNAs), which are over 200 nucleotides have been greatly studied in multiple diseases and biology [19]. The functions and mechanisms of lncRNAs in mammalian cells are described in detail in Figure 1 [20–32]. Moreover, lncRNAs are involved in cancer biology and have been listed into one of the hallmarks of cancer [33, 34]. They are associated with oncogenesis and may serve as promising a new type of biomarkers for tumor diagnosis, prognosis, even in targeted gene therapy [19, 35, 36].
Figure 1. The functions and mechanisms of long non-coding RNAs.
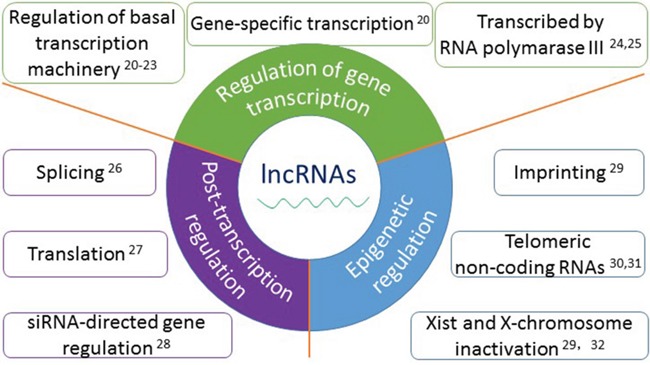
Accumulated evidence show that lncRNAs play important roles in a wide variety of biological processes, mainly in three aspects: 1. In the regulation of gene transcription, such as regulation of basal transcription machinery, gene-specific transcription and transcribed by RNA polymerase III; 2. In post-transcriptional regulation, such as splicing, translation, and siRNA-directed gene regulation; 3. In epigenetic regulation, such as imprinting, telomeric non-coding RNAs and Xist and X-chromosome inactivation.
LncRNAs have been found involved in tumorigenesis, disease progression and metastasis of RCC. They function as oncogenes or tumor suppress genes and regulate multiple biologic or pathological processes, which are shown in Figure 2. But the underlying mechanisms still need to be further explored. In this systematic review, we focus on the expression profile of lncRNAs in RCC, with emphasize on the roles of oncogenesis, diagnosis, progression, prognosis and the potential of application in RCC therapy.
Figure 2. The functions of lncRNAs in pathogenesis and potential clinical applications in RCC.
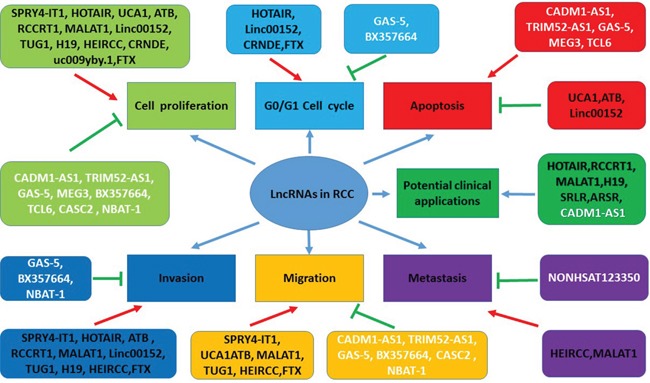
LncRNAs play multiple functions in the context of RCC, including regulating cell proliferation, cell cycle, apoptosis, invasion, migration, and metastasis in the forms of oncogenes or tumor suppressor genes. Some lncRNAs maybe serve as therapeutic targets and have the potential of the clinical application. Red arrows indicate promoted signaling pathways. Green arrows indicate inhibited signaling pathways. LncRNAs in black font indicate oncogenes. LncRNAs in white font indicate tumor suppressor genes. Some lncRNAs have multiple functions (for example, MALAT1 promotes cell proliferation, invasion, migration, and metastasis). Abbreviations: SPRY4-IT1: SPRY4 intronic transcript 1; HOTAIR: Hox transcript antisense intergenic RNA; UCA1: urothelial carcinoma-associated 1; lncRNA-ATB: lncRNA activated by TGF-β; MALAT1: metastasis-associated lung adenocarcinoma transcript 1; Linc00152: long intergenic noncoding RNA 152; TUG1: Taurine up-regulated gene 1; HEIRCC: high-expressed in renal cell carcinoma, lncRNA TCONS_00006756; CRNDE: colorectal neoplasia differentially expressed; CADM1-AS1: lncRNA cell adhesion molecule 1 antisense, lncRNA (RNA176206/ENST00000546273) located in the antisense direction of a coding exon of the cell adhesion molecule1 (CADM1); TRIM52-AS1: TRIM52 antisense RNA 1; GAS-5: growth arrest specific 5; MEG3: maternally expressed gene 3; TCL6: T-cell leukemia/lymphoma 6; CASC2: cancer susceptibility candidate 2; NBAT-1: neuroblastoma associated transcript 1; SRLR: sorafenib resistance-associated lncRNA in RCC; ARSR: lncRNA activated in RCC with sunitinib resistance, ENST00000424980.
LNCRNAS IN RCC
Aberrant expression profiling of lncrnas in RCC
Many studies about lncRNAs expression profiling have been carried out in RCC. The determination of their interaction with other molecules and functional analysis is also booming in recent years. The most popular methods for the study of lncRNAs expression profiles of RCC are microarray assay and ChIP-Seq in small sample trials [37–43]. If certain lncRNAs are found to be obviously dysregulated, following qPCR experiments to demonstrate the significance in large scale samples are preferred, some of these significant dysregulated lncRNAs confirmed by qPCR may even serve as a diagnostic or prognostic biomarkers. [37–39]. A couple of recent studies found that hundreds of thousands of lncRNAs were aberrantly expressed in RCC tissue compared with adjacent non-tumor tissue through genome wide assay [37–43]. We summarized these studies in Table 1 in detail [37–43]. In addition, lncRNAs deregulated in RCC are shown in Table 2A and Table 2B; the functions, targeted genes/signaling, and the mechanisms involved are also indicated [44–67].
Table 1. The studies about expression profiling of lncRNAs in RCC.
| Author | Year | Country | RCC subtype | Samplesize (n) | Differentiallyexpressed lncRNAs | Numberupregulated | Numberdownregulated | References |
|---|---|---|---|---|---|---|---|---|
| Yu et al | 2012 | China | ccRCC | 6 | 726 | 146 | 480 | 37 |
| Liu et al | 2016 | China | not mentioned | 90 | 3862 | 1649 | 2243 | 38 |
| Deng, Blondeau et al | 2015 | Germany | ccRCC | 15 | 1308 | 568 | 740 | 39, 40 |
| Qin et al | 2014 | China | ccRCC | 5 | 897 | 480 | 417 | 41 |
| Fachel et al | 2013 | Brazil | ccRCC | 11 | 40 | 14 | 26 | 42 |
| He et al | 2016 | China | chRCC | 59 | 143 | 41 | 102 | 43 |
Table 2A. Upregulated lncRNAs in RCC.
| lncRNAs | Specimens | Functions | Target genes/Signalings | Pathways/Mechanisms involved | Referances |
|---|---|---|---|---|---|
| SPRY4-IT1 | RCC tissues, cell lines | oncogene | proliferation, migration, invasion | 44 | |
| HOTAIR | cell lines | oncogene | H3K27me, EZH2, miR-141, Ago2 | proliferation, invasion, cell cycle | 45, 46 |
| UCA1 | RCC tissues, cell lines | oncogene | proliferation, migration, apoptosis | 47 | |
| lncRNA-ATB | RCC tissues, cell lines | oncogene | EMT | proliferation, apoptosis, migration, invasion | 48 |
| RCCRT1 | RCC tissues | biomarker | migration, invasion | 49 | |
| MALAT1 | RCC tissues, cell lines | oncogene | Ezh2, miR-205 | proliferation, migration, invasion | 50, 51 |
| Linc00152 | RCC tissues, cell lines | oncogene, biomarker | proliferation, invasion, apoptosis, cell cycle | 52 | |
| TUG1 | RCC tissues, cell lines | oncogene | migration, invasion, proliferation, apoptosis | 53 | |
| H19 | RCC tissues, cell lines | biomarker | proliferation, invasion, migration | 54 | |
| HEIRCC | RCC tissues, cell lines | oncogene | EMT | proliferation, apoptosis, migration, invasion | 55 |
| CRNDE | RCC tissues, cell lines | oncogene | Wnt/β-catenin signaling | proliferation, growth, cell cycle | 56 |
| uc009yby.1 | RCC tissues | oncogene | proliferation | 57 | |
| FTX | RCC tissues, cell lines | oncogene | proliferation, cell cycle, migration, invasion | 58 | |
| PVT1 | RCC tissues | oncogene | MYC | promoter hypomethylation | 59 |
Table 2B. Downregulated lncRNAs in RCC.
| lncRNAs | Specimens | Functions | Target genes/Signalings | Pathways/Mechanisms involved | Referances |
|---|---|---|---|---|---|
| CADM1-AS1 | RCC tissues | tumor suppressor | CADM1 | cell proliferation, apoptosis and migration | 60 |
| TRIM52-AS1 | RCC tissues | tumor suppressor | proliferation, cell migration and apoptosis | 61 | |
| GAS-5 | RCC tissues | tumor suppressor | proliferation, apoptosis, cell cycle, migration, invasion | 62 | |
| MEG3 | RCC tissues, cell lines | tumor suppressor | Bcl-2, rocaspase-9, leaved caspase-9, cytochrome c | apoptosis, mitochondrial pathway | 63 |
| BX357664 | RCC tissues, cell lines | tumor suppressor | EMT, MMP2, MMP9, TGF-β1/p38/HSP27 | proliferation, migration, invasion, cell cycle | 64 |
| TCL6 | RCC tissues | tumor suppressor | proliferation, apoptosis | 65 | |
| CASC2 | RCC tissues, cell lines | tumor suppressor | miR-21 | proliferation, migration | 66 |
| NBAT-1 | RCC tissues, cell lines | prognostic biomarker | proliferation, migration, invasion | 67 |
The lncRNAs aberrantly expressed maybe participate in the carcinogenesis and progression of RCC. In addition, lncRNA profiling and the research results indicate that lncRNAs may be suitable for diagnostic, even in the prediction of prognostic purposes for RCC. A deeper study of these lncRNAs may pave a way for a further understanding in the basic science of RCC. Despite numerous efforts have been made, the mechanisms of lncRNAs, such as the transduction pathways and regulation functions of lncRNAs in RCC still need to be explored in further detail.
Diagnosis and biomarkers
Many studies of determining aberrant lncRNAs expression for diagnostic purpose and the identification of novel deregulated lncRNAs as biomarkers for RCC have been carried out. A recent study found that the expression level of CYP4A22-2/3 can discriminate ccRCCs from normal kidney tissues [68]. In another study, lncRNA-ATB was found to be elevated in metastatic RCC patients, and higher expression of lncRNA-ATB correlated with disease progression and a more invasive feature, as well as metastasis [48]. In addition, RCCRT1 has a significant relationship with clinicopathologic features of ccRCC patients, including the size of tumor, the pathological staging and tumor grade [49], it is also associated with metastasis of lymph node as well as distal metastasis in ccRCC [49]. Moreover, TCL6 expression was an independent predictor of ccRCC aggressiveness and was negatively correlated with the T, N, M, and TNM stage [65].
The technique for detection and measurement of specific serum lncRNAs has become feasible, which provides a new method without invasiveness for the identification of new biomarker for cancer. Recent studies found that serum lncRNAs are useful for the diagnosis of multiple types of cancer, such as bladder cancer [69], liver cancer [70], cervical cancer [71], gastric cancer [72], colorectal cancer [73, 74] and breast cancer [75] etc, these serum lncRNAs may even serve as prognostic biomarkers [69, 72, 74]. Serum lncRNAs in RCC patients are also deregulated. Wu et al performed a study that demonstrated that five lncRNAs are significantly dysregulated in the serum of RCC patients-lncRNAs (LET, PVT1, PANDAR, PTENP1, and linc00963)- this 5 lncRNAs serum signature may obtain a preferable sensitivity and specificity for distinguishing ccRCC patients from normal individuals [76].
Prognosis
A multitude of studies have shown that aberrant lncRNA expressions are associated with the disease overall survival (OS), the 5-year survival, the disease-free survival (DFS), the disease grade and stage, recurrence, metastasis etc. As shown in Table 3, decreased expression of lncRNA NONHSAT123350, CADM1-AS1, TCL6 and lnc-ZNF180-2 is correlated with poorer prognosis of RCC patients [38, 60, 65, 68]. On the other hand, overexpression of SPRY4-IT1, RCCRT1, MALAT1, Linc00152 and PVT1 shows a poor prognosis, as well [44, 49–52, 59].
Table 3. Prognostic lncRNAs in RCC.
| lncRNAs | Prognostic information | Referances |
|---|---|---|
| CADM1-AS1 | decreased expression, poor prognosis (OS) | 60 |
| TCL6 | low expression, poor prognosis (OS) | 65 |
| NONHSAT123350 | low expression, poor prognosis (DFS, OS) | 38 |
| lnc-ZNF180-2 | low level, poor progression-free, CSS and OS | 68 |
| SPRY4-IT1 | high expression, poor prognosis (OS) | 44 |
| RCCRT1 | high expression, poor survival | 49 |
| MALAT1 | high expression, poor prognosis (OS) | 50 |
| Linc00152 | high expression, poor prognosis (OS) | 52 |
| DRAIC | overexpression, good prognosis | 77 |
| PVT1 | high expression, poor prognosis (OS) | 59 |
The expression level of lncRNA NONHSAT123350 was closely associated with OS and DFS in patients without distant metastasis; the median values of OS and DFS were significantly higher among patients with high NONHSAT123350 expression when compared to the patients whose expression of NONHSAT123350 were low [38]. In another study, Ellinger et al reported the expression of lnc-ZNF180-2 significantly increased in advanced ccRCC tissue compared with localized ccRCC and normal renal tissue; a shorter period of progression-free survival (PFS), cancer-specific survival (CSS), and OS following nephrectomy happened in patients with lower levels of lnc-ZNF180-2 in the univariate Cox regression analysis. Thereafter, lnc-ZNF180-2 expression levels showed an independent predictor of progression-free survival (PSF), CSS, and OS in ccRCC patients within the multivariate Cox regression model [68]. In addition, the ccRCC patients had an advanced clinical stage and poorer prognosis when the SPRY4-IT1 expression was at high level. It is identified that SPRY4-IT1 is an independent prognostic factor in ccRCC in Cox proportional hazard model [44]. DRAIC is a new tumor suppressive sequence which locates on chromosome 15q23. DRAIC lncRNA was identified downregulated as prostate cancer cells progress from an androgen dependent to castration resistant state. DRAIC overexpression indicates a favorable prognosis in many kinds of malignancies including RCC [77]. Recently, a study showed that high Linc00152 expression was closely associated with advanced TNM stage in RCC patients. Moreover, Linc00152 is able to serve as an independent predictor of OS for the patients [52]. In addition, eight lncRNAs transcribed from the loci (ACTN4, CSNK1D, DNAJC3, GIGYF2, HDAC5, PTPN3, RAB25, and VPS13B) were detected as altered in the expression profiles in both of the malignancy and the survival outcomes [42]. Furthermore, PVT1 was strongly overexpressed in ccRCC and associated with the enhancement of MYC signaling and poorer clinical outcome [57].
Therapy
Good ways to study the potential therapeutic function in cancer include lncRNAs are through loss-of-function or gain-of-function, RNA interfering technology, genetic loss-of-function models, or overexpression [33]. Therapeutic silencing or overexpression of these lncRNAs might be a viable therapeutic option to reduce the growth and/or metastatic potential of RCC. LncRNAs that may serve as therapeutic targets for RCC are listed in Table 4 and discussed as below.
Table 4. LncRNAs potentially serve as therapeutic targets for RCC.
| LncRNAs | Location | Properties/Mechanisms | References |
|---|---|---|---|
| HOTAIR | chromosome 12 | recruit and bind on the locus of EZH2 and H3K27me3 | 80 |
| inhibit cycle-related genes p53, p21 and p16 | 80 | ||
| modulate covalent histones | 80 | ||
| interact with methyltransferase PRC2, histone demethylase LSD1 | 80 | ||
| regulates gene silencing | 80 | ||
| required for H3K27 trimethylation | 81 | ||
| transcriptional silencing across the HOXD locus | 81 | ||
| RCCRT1 | chr5:137801181-137805004 | upregulated in RCC | 49 |
| upregulated predicts poor survival of RCC | 49 | ||
| MALAT1 | chromosome 11q13 | independent predictor of OS in ccRCC | 50, 51 |
| sequester serine/arginine splicing factors in nuclear speckle domains | 83, 84 | ||
| regulate alternative splicing | 84 | ||
| transcriptional activation of MALAT1 by c-Fos contribute to oncogenesis | 51 | ||
| interact with Ezh2 | 51 | ||
| reciprocally repressed with miR205 | 51 | ||
| regulate EMT via E-cadherin and β-catenin | 51 | ||
| promote ZEB2 expression by sponging miR-200s | 85 | ||
| H19 | 11p15.5 | exon 1 of H19 harbors a miRNA-containing hairpin | 86 |
| serve as the template of two miR6755p and miR6753p | 86 | ||
| LncRNA-SRLR | 3q24 | upregulated in intrinsically sorafenib resistant RCC | 87 |
| LncARSR | 9q82.120.717-82.185.824 | promote sunitinib resistance of RCC | 88 |
| competitively bind to miR-34/miR-449 | 88 | ||
| facilitate AXL and c-MET expression | 88 | ||
| exosome-transferred lncARSR confer sunitinib resistance | 88 | ||
| targeting lncARSR restores sunitinib response in RCC | 88 | ||
| CADM1-AS1 | antisense direction of a coding exon ofthe cell adhesion molecule 1 (CADM1) | involved in renal carcinogenesisindependent prognostic factor for ccRCC |
60 60 |
HOTAIR
HOTAIR is transcribed from antisense strand of HOXC gene cluster present in chromosome 12 with a length of 2.2kb [78]. Recent study find that it can regulate EMT and is involved in Notch pathway [79]. Moreover, HOTAIR is a target of miR-141, inhibiting HOTAIR function in an Ago2-dependent manner [46]. Through RNAi technology, HOTAIR knockdown can affect cell cycle in G0/G1 phase and decrease cell proliferation and invasion of RCC cells [45]. The inhibiton of HOTAIR also suppressed tumor formation in the xenograft experiments in vivo [45]. Further studies are urgent for determining the regulatory mechanisms of HOTAIR, together with the interactions with epigenetic processes, such as DNA methylation, histone modification, microRNA and ubiquitin, by which new therapeutic targets might be developed in the treatment of RCC.
RCCRT1
RCCRT1 is upregulated in RCC compared with tumor adjacent tissue, particularly in high-grade RCC tissue. It is considered to be an oncogene of RCC, and elevated expression of RCCRT1 may predict dismal survival of RCC patients [49]. The knockdown of RCCRT1 by RNAi technique can suppress migration and invasion in RCC cell lines [49].
MALAT1
MALAT1 also called nuclear-enriched abundant transcript 2 (NEAT2) or Mhrt, it is one of the up-regulated nuclear lncRNAs in mammals [82]. The expression of MALAT1 is found to be upregulated in RCC tissues compared with corresponding normal tissues [50, 51]. Over-expression of MALAT1 indicates a more aggressive feature of the tumor and predicts a poorer OS than down-expression of MALAT1 in patients with ccRCC [50, 51]. In vitro experiments found that the inhibition of MALAT1 not only suppressed cell proliferation, promoted apoptosis, but also inhibited migration, and invasion of RCC cells [50, 51]. Thus, inhibition of MALAT1 may become a promising strategy for RCC therapy.
H19
The lncRNA H19 is another well-known oncogene in various cancer types, tumorigenesis, and cancer progression. It is transcribed from the sequence localized at 11p15.5 of the human genome. It is associate with disease progression and poorer prognosis of RCC patients [54]. Moreover, inhibition of H19 can suppress proliferation, invasion, and migration of RCC cells [54].
CADM1-AS1
LncRNA CADM1-AS is considered to be involved in the carcinogenesis of RCC. It is found to be decreased in RCC tissues [60]. Down-regulation of CADM1-AS1 correlates with advanced disease staging and poor prognosis of patients with ccRCC [60]. Over-expression of CADM1-AS1 can significantly decrease cell growth and migration, as well as increase apoptosis in RCC cells [60]. Therefore, CADMA1-AS1 maybe become a suitable therapeutic marker for RCC.
LncRNA-SRLR and lncARSR
Targeted therapy drugs, such as sorafenib and sunitinib, appear to increase the survival rate of RCC patients. But targeted therapy resistance is a major obstacle for the treatment of advanced and/or metastatic RCC. LncRNA-SRLR (sorafenib resistance-associated lncRNA in RCC) was found upregulated in intrinsically sorafenib resistant RCCs. Interestingly, lncRNA-SRLR knockdown sensitized nonresponsive RCC cells to sorafenib treatment, whereas the overexpression of lncRNA-SRLR conferred sorafenib resistance to responsive RCC cells. The underlying mechanism is that lncRNA-SRLR directly binds to NF-κB and promotes IL-6 transcription, leading to the activation of STAT3 and the development of sorafenib tolerance. A STAT3 inhibitor and IL-6-receptor antagonist both restored the response to sorafenib treatment. Moreover, high levels of lncRNA-SRLR correlated with poor responses to sorafenib therapy in RCC patients [87]. LncARSR (activated in RCC with sunitinib resistance, ENST00000424980) was a newly identified lncRNA to promote the sunitinib resistance of RCC. It is upregulated in sunitinib resistant RCC. By targeting lncARSR and the underlining pathways involved, sunitinib resistance RCC can restore the sensitivity to sunitinib [88]. Another study showed that lncARSR was upregulated in primary renal cancer stem cells (CSCs) and was associated with a poor prognosis of ccRCC. Knockdown of lncARSR attenuated the self-renewal, tumorigenicity, and metastasis of renal CSCs. Conversely, forced lncARSR expression enhanced CSC properties of RCC cells. The mechanism showed that the binding of lncARSR to Yes-associated protein (YAP) impedes LATS1-induced YAP phosphorylation and facilitates YAP nuclear translocation [89].
These findings are extremely meaningful, because lncRNAs may act as predictors and potential promising therapeutic targets for target therapy resistance, and may further improve the management of RCC patients receiving target therapy. If these lncRNAs could be applied to the treatment of RCC, it would benefit the patients, not only on efficacy, but also economically. Hence, further studies should be made for the identification of lncRNAs as targeted therapy biomarkers and application for RCC treatment strategy.
Specific molecular mechanism of lncrnas in RCC
Hypoxia-inducible factor-2α (HIF2A) signaling participates in the RCC oncogenesis and progression as reported [90]. In this pathway, LncRNA-suppressing androgen receptor in renal cell carcinoma (SARCC) serves as a critical regulator, it connects with the AR/HIF2A/MYC axis to regulate cell growth in response to hypoxia. LncRNA-SARCC suppresses hypoxic cell cycle progression in VHL mutant type of RCC cells and de-represses it in VHL restored type of RCC cells. Mechanistically, lncRNA-SARCC binds to and deactivates androgen receptor (AR) protein to modulate AR post transcription thus block the AR/HIF2A/ MYC signals; on the other hand, HIF2A is able to regulate the lncRNA-SARCC expression on the transcription level by binding to hypoxia responsive elements on the lncRNA-SARCC promoter vice versa [90]. There is a negative feedback regulation loop between complex of LncRNA-SARCC/AR and HIF2A pathway, by which may result a differentially regulated RCC disease progression in a VHL dependent way [90]. Thereafter, future studies should be done to identify the association of lncRNAs and HIF signals in RCC.
Interestingly, a recent study from China established a novel method designed to identify lncRNA competitively regulated signal subpathways, so called subpathway-LNCE, which can identify lncRNA competitively regulated functions. The functional roles of these competitive regulation lncRNAs have not be well characterized in diseases. Moreover, the method integrated lncRNA-mRNA expression profile and pathway topologies [91].
CONCLUSIONS
In conclusion, lncRNAs are aberrantly expressed in RCC, and participate in important roles in the regulation of RCC. They have a significant impact on our understanding the mechanisms of pathogenesis, progression and metastasis of RCC. They are also involve in key pathological process in RCC, the cell proliferation, cell cycle control, apoptosis, local invasion and distal metastasis. To deeply identify the mechanisms regarding how specific lncRNAs affect RCC and the regulatory mechanisms, further exploration and continued studies are required. At the same time, the lncRNA transduction pathway in RCC still needs to be further explored. Moreover, lncRNAs may act as promising new biomarkers for RCC, with the capability of improving diagnosis, predicting prognosis, and even in the improvement of therapeutic strategy for RCC patients. Several newly identified lncRNAs have the function of predicting target therapy resistance, by targeting these lncRNAs, the resistance of target therapies may be restored their sensitivity. RCC is an immunotherapy effective disease and the determination of whether lncRNAs could regulate the immune pathways of RCC such as PD-1 or PD-L1 signaling are expected. In any cases, to deeply understand the mechanisms of lncRNAs in RCC can not only help to uncover the tumorigenesis, but also have the possibility of application in the clinical management.
Acknowledgments
This work was supported by the Promising Talents Plan Program Funding of Shengjing Hospital, China Medical University. The authors are very grateful to the reviewers and editors, without their help, we could not complete this work. We apologize to those whose research we could not cite due to the limitations of our topic and space.
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Vera-Badillo FE, Conde E, Duran I. Chromophobe renal cell carcinoma: A review of an uncommon entity. Int J Urol. 2012;19:894–900. doi: 10.1111/j.1442-2042.2012.03079.x. [DOI] [PubMed] [Google Scholar]
- 4.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol. 2016;70:93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–9. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Cristofano C, Minervini A, Menicagli M, Salinitri G, Bertacca G, Pefanis G, Masieri L, Lessi F, Collecchi P, Minervini R, Carini M, Bevilacqua G, Cavazzana A. Nuclear expression of hypoxia-inducible factor-1alpha in clear cell renal cell carcinoma is involved in tumor progression. Am J Surg Pathol. 2007;31:1875–81. doi: 10.1097/PAS.0b013e318094fed8. [DOI] [PubMed] [Google Scholar]
- 7.Elfiky AA, Aziz SA, Conrad PJ, Siddiqui S, Hackl W, Maira M, Robert CL, Kluger HM. Characterization and targeting of phosphatidylinositol-3 kinase (PI3K) and mammalian target of rapamycin (mTOR) in renal cell cancer. J Transl Med. 2011;9:133. doi: 10.1186/1479-5876-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chappell WH, Steelman LS, Long JM, Kempf RC, Abrams SL, Franklin RA, Bäsecke J, Stivala F, Donia M, Fagone P, Malaponte G, Mazzarino MC, Nicoletti F, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR inhibitors: rationale and importance to inhibiting these pathways in human health. Oncotarget. 2011;2:135–65. doi: 10.18632/oncotarget.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shang D, Liu Y, Ito N, Kamoto T, Ogawa O. Defective Jak-Stat activation in renal cell carcinoma is associated with interferon-alpha resistance. Cancer Sci. 2007;98:1259–64. doi: 10.1111/j.1349-7006.2007.00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Q, Krause M, Samoylenko A, Vainio S. Wnt Signaling in Renal Cell Carcinoma. Cancers (Basel) 2016;8 doi: 10.3390/cancers8060057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Wang Y, Song Y, Bu R, Yin B, Fei X, Guo Q, Wu B. Expression profiling and clinicopathological significance of DNA methyltransferase 1, 3A and 3B in sporadic human renal cell carcinoma. Int J Clin Exp Pathol. 2014;7:7597–609. [PMC free article] [PubMed] [Google Scholar]
- 12.Mosashvilli D, Kahl P, Mertens C, Holzapfel S, Rogenhofer S, Hauser S, Buttner R, Von Ruecker A, Muller SC, Ellinger J. Global histone acetylation levels: prognostic relevance in patients with renal cell carcinoma. Cancer Sci. 2010;101:2664–9. doi: 10.1111/j.1349-7006.2010.01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Wang Y, Song Y, Bu R, Yin B, Fei X, Guo Q, Wu B. MicroRNAs in renal cell carcinoma: a systematic review of clinical implications (Review) Oncol Rep. 2015;33:1571–8. doi: 10.3892/or.2015.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hangauer MJ, Vaughn IW, McManus MT. Pervasive transcription of the human genome produces thousands of previously unidentified long intergenic noncoding RNAs. PLoS Genet. 2013;9:e1003569. doi: 10.1371/journal.pgen.1003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Consortium EP. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bierhoff H, Schmitz K, Maass F, Ye J, Grummt I. Noncoding transcripts in sense and antisense orientation regulate the epigenetic state of ribosomal RNA genes. Cold Spring Harb Symp Quant Biol. 2010;75:357–64. doi: 10.1101/sqb.2010.75.060. [DOI] [PubMed] [Google Scholar]
- 17.Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, Cabili MN, Jaenisch R, Mikkelsen TS, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–7. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St Laurent G, Wahlestedt C, Kapranov P. The Landscape of long noncoding RNA classification. Trends Genet. 2015;31:239–51. doi: 10.1016/j.tig.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt AM, Chang HY. Long Noncoding RNAs in Cancer Pathways. Cancer Cell. 2016;29:452–63. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodrich JA, Kugel JF. Non-coding-RNA regulators of RNA polymerase II transcription. Nat Rev Mol Cell Biol. 2006;7:612–6. doi: 10.1038/nrm1946. [DOI] [PubMed] [Google Scholar]
- 21.Kwek KY, Murphy S, Furger A, Thomas B, O'Gorman W, Kimura H, Proudfoot NJ, Akoulitchev A. U1 snRNA associates with TFIIH and regulates transcriptional initiation. Nat Struct Biol. 2002;9:800–5. doi: 10.1038/nsb862. [DOI] [PubMed] [Google Scholar]
- 22.Mariner PD, Walters RD, Espinoza CA, Drullinger LF, Wagner SD, Kugel JF, Goodrich JA. Human Alu RNA is a modular transacting repressor of mRNA transcription during heat shock. Mol Cell. 2008;29:499–509. doi: 10.1016/j.molcel.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Martianov I, Ramadass A, Serra Barros A, Chow N, Akoulitchev A. Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature. 2007;445:666–70. doi: 10.1038/nature05519. [DOI] [PubMed] [Google Scholar]
- 24.Dieci G, Fiorino G, Castelnuovo M, Teichmann M, Pagano A. The expanding RNA polymerase III transcriptome. Trends Genet. 2007;23:614–22. doi: 10.1016/j.tig.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Pagano JM, Farley BM, McCoig LM, Ryder SP. Molecular basis of RNA recognition by the embryonic polarity determinant MEX-5. J Biol Chem. 2007;282:8883–94. doi: 10.1074/jbc.M700079200. [DOI] [PubMed] [Google Scholar]
- 26.Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, Blencowe BJ, Prasanth SG, Prasanth KV. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–38. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majidinia M, Mihanfar A, Rahbarghazi R, Nourazarian A, Bagca B, Avci CB. The roles of non-coding RNAs in Parkinson's disease. Mol Biol Rep. 2016;43:1193–204. doi: 10.1007/s11033-016-4054-3. [DOI] [PubMed] [Google Scholar]
- 28.Czech B, Malone CD, Zhou R, Stark A, Schlingeheyde C, Dus M, Perrimon N, Kellis M, Wohlschlegel JA, Sachidanandam R, Hannon GJ, Brennecke J. An endogenous small interfering RNA pathway in Drosophila. Nature. 2008;453:798–802. doi: 10.1038/nature07007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pauler FM, Koerner MV, Barlow DP. Silencing by imprinted noncoding RNAs: is transcription the answer? Trends Genet. 2007;23:284–92. doi: 10.1016/j.tig.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoeftner S, Blasco MA. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat Cell Biol. 2008;10:228–36. doi: 10.1038/ncb1685. [DOI] [PubMed] [Google Scholar]
- 31.Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J. Telomeric repeat- containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318:798–801. doi: 10.1126/science.1147182. [DOI] [PubMed] [Google Scholar]
- 32.Furlan G, Rougeulle C. Function, and evolution of the long noncoding RNA circuitry orchestrating X-chromosome inactivation in mammals. Wiley Interdiscip Rev RNA. 2016;7:702–22. doi: 10.1002/wrna.1359. [DOI] [PubMed] [Google Scholar]
- 33.Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9:703–19. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–23. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Chen Y, Gu L, Li X, Gao Y, Lyu X, Chen L, Luo G, Wang L, Xie Y, Duan J, Peng C, Ma X. LncRNAs act as prognostic and diagnostic biomarkers in renal cell carcinoma a systematic review and meta-analysis. Oncotarget. 2016;7:74325–74336. doi: 10.18632/oncotarget.11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z, Chen Y, Hu S, Zhang J, Wu J, Ren W, Shao N, Ying X. Integrative analysis of protein-coding and non-coding RNAs identifies clinically relevant subtypes of clear cell renal cell carcinoma. Oncotarget. 2016;7:82671–82685. doi: 10.18632/oncotarget.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu G, Yao W, Wang J, Ma X, Xiao W, Li H, Xia D, Yang Y, Deng K, Xiao H, Wang B, Guo X, Guan W, et al. LncRNAs expression signatures of renal clear cell carcinoma revealed by microarray. PLoS One. 2012;7:e42377. doi: 10.1371/journal.pone.0042377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu H, Chen P, Jiang C, Han J, Zhao B, Ma Y, Mardan M. Screening for the Key lncRNA Targets Associated With Metastasis of Renal Clear Cell Carcinoma. Medicine (Baltimore) 2016;95:e2507. doi: 10.1097/MD.0000000000002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng M, Blondeau JJ, Schmidt D, Perner S, Muller SC, Ellinger J. Identification of novel differentially expressed lncRNA and mRNA transcripts in clear cell renal cell carcinoma by expression profiling. Genom Data. 2015;5:173–5. doi: 10.1016/j.gdata.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blondeau JJ, Deng M, Syring I, Schroder S, Schmidt D, Perner S, Muller SC, Ellinger J. Identification of novel long non-coding RNAs in clear cell renal cell carcinoma. Clin Epigenetics. 2015;7:10. doi: 10.1186/s13148-015-0047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castresana JS, Qin C, Han Z, Qian J, Bao M, Li P, Ju X, Zhang S, Zhang L, Li S, Cao Q, Lu Q, Li J, et al. Expression Pattern of Long Non-Coding RNAs in Renal Cell Carcinoma Revealed by Microarray. PLoS ONE. 2014;9:e99372. doi: 10.1371/journal.pone.0099372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fachel AA, Tahira AC, Vilella-Arias SA, Maracaja-Coutinho V, Gimba ER, Vignal GM, Campos FS, Reis EM, Verjovski-Almeida S. Expression analysis and in silico characterization of intronic long non-coding RNAs in renal cell carcinoma: emerging functional associations. Mol Cancer. 2013;12:140. doi: 10.1186/1476-4598-12-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He HT, Xu M, Kuang Y, Han XY, Wang MQ, Yang Q. Biomarker and competing for endogenous RNA potential of tumor-specific long noncoding RNA in chromophobe renal cell carcinoma. Onco Targets Ther. 2016;9:6399–6406. doi: 10.2147/OTT.S116392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang HM, Yang FQ, Yan Y, Che JP, Zheng JH. High expression of long non-coding RNA SPRY4-IT1 predicts poor prognosis of clear cell renal cell carcinoma. Int J Clin Exp Pathol. 2014;7:5801–9. [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Y, Liu J, Zheng Y, You L, Kuang D, Liu T. Suppressed expression of long non-coding RNA HOTAIR inhibits proliferation and tumourigenicity of renal carcinoma cells. Tumour Biol. 2014;35:11887–94. doi: 10.1007/s13277-014-2453-4. [DOI] [PubMed] [Google Scholar]
- 46.Chiyomaru T, Fukuhara S, Saini S, Majid S, Deng G, Shahryari V, Chang I, Tanaka Y, Enokida H, Nakagawa M, Dahiya R, Yamamura S. Long non-coding RNA HOTAIR is targeted and regulated by miR-141 in human cancer cells. J Biol Chem. 2014;289:12550–65. doi: 10.1074/jbc.M113.488593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Wang T, Li Y, Chen D, Yu Z, Jin L, Ni L, Yang S, Mao X, Gui Y, Lai Y. Identification of long-non-coding RNA UCA1 as an oncogene in renal cell carcinoma. Mol Med Rep. 2016;13:3326–34. doi: 10.3892/mmr.2016.4894. [DOI] [PubMed] [Google Scholar]
- 48.Xiong J, Liu Y, Jiang L, Zeng Y, Tang W. High expression of long non-coding RNA lncRNA-ATB is correlated with metastases and promotes cell migration and invasion in renal cell carcinoma. Jpn J Clin Oncol. 2016;46:378–84. doi: 10.1093/jjco/hyv214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song S, Wu Z, Wang C, Liu B, Ye X, Chen J, Yang Q, Ye H, Xu B, Wang L. RCCRT1 is correlated with prognosis and promotes cell migration and invasion in renal cell carcinoma. Urology. 2014;84:730 e1–7. doi: 10.1016/j.urology.2014.05.033. [DOI] [PubMed] [Google Scholar]
- 50.Zhang HM, Yang FQ, Chen SJ, Che J, Zheng JH. Upregulation of long non-coding RNA MALAT1 correlates with tumor progression and poor prognosis in clear cell renal cell carcinoma. Tumour Biol. 2015;36:2947–55. doi: 10.1007/s13277-014-2925-6. [DOI] [PubMed] [Google Scholar]
- 51.Hirata H, Hinoda Y, Shahryari V, Deng G, Nakajima K, Tabatabai ZL, Ishii N, Dahiya R. Long Noncoding RNA MALAT1 Promotes Aggressive Renal Cell Carcinoma through Ezh2 and Interacts with miR-205. Cancer Res. 2015;75:1322–31. doi: 10.1158/0008-5472.CAN-14-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Y, Tan C, Weng WW, Deng Y, Zhang QY, Yang XQ, Gan HL, Wang T, Zhang PP, Xu MD, Wang YQ, Wang CF. Long non-coding RNA Linc00152 is a positive prognostic factor for and demonstrates malignant biological behavior in clear cell renal cell carcinoma. Am J Cancer Res. 2016;6:285–99. [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang M, Lu W, Huang Y, Shi J, Wu X, Zhang X, Jiang R, Cai Z, Wu S. Downregulation of the long noncoding RNA TUG1 inhibits the proliferation, migration, invasion and promotes apoptosis of renal cell carcinoma. J Mol Histol. 2016;47:421–8. doi: 10.1007/s10735-016-9683-2. [DOI] [PubMed] [Google Scholar]
- 54.Wang L, Cai Y, Zhao X, Jia X, Zhang J, Liu J, Zhen H, Wang T, Tang X, Liu Y, Wang J. Down-regulated long non-coding RNA H19 inhibits carcinogenesis of renal cell carcinoma. Neoplasma. 2015;62:412–8. doi: 10.4149/neo_2015_049. [DOI] [PubMed] [Google Scholar]
- 55.Xiong J, Liu Y, Luo S, Jiang L, Zeng Y, Chen Z, Shi X, Lv B, Tang W. High expression of the long non-coding RNA HEIRCC promotes Renal Cell Carcinoma metastasis by inducing epithelial-mesenchymal transition. Oncotarget. 2017;8:6555–6563. doi: 10.18632/oncotarget.14149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shao K, Shi T, Yang Y, Wang X, Xu D, Zhou P. Highly expressed lncRNA CRNDE promotes cell proliferation through Wnt/β-catenin signaling in renal cell carcinoma. Tumour Biol. 2016 Oct;6 doi: 10.1007/s13277-016-5440-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 57.Ren X, Lan T, Chen Y, Shao Z, Yang C, Peng J. lncRNA uc009yby.1 promotes renal cell proliferation and is associated with poor survival in patients with clear cell renal cell carcinomas. Oncol Lett. 2016;12:1929–1934. doi: 10.3892/ol.2016.4856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.He X, Sun G, Guo F, Wang K, Gao Y, Feng Y, Song B, Li W, Li Y. Knockdown of long non-coding RNA FTX inhibits proliferation, migration, and invasion in renal cell carcinoma cells. Oncol Res. 2016 Oct;26 doi: 10.3727/096504016×14719078133203. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Posa I, Carvalho S, Tavares J, Grosso AR. A pan-cancer analysis of MYC-PVT1 reveals CNV-unmediated deregulation and poor prognosis in renal carcinoma. Oncotarget. 2016;7:47033–47041. doi: 10.18632/oncotarget.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao J, Chen Y, Wang Y, Liu S, Yuan X, Pan F, Geng P. Decreased expression of a novel lncRNA CADM1-AS1 is associated with poor prognosis in patients with clear cell renal cell carcinomas. Int J Clin Exp Pathol. 2014;7:2758–67. [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Z, Yan HY, Xia SY, Zhang C, Xiu YC. Downregulation of long non-coding RNA TRIM52-AS1 functions as a tumor suppressor in renal cell carcinoma. Mol Med Rep. 2016;13:3206–12. doi: 10.3892/mmr.2016.4908. [DOI] [PubMed] [Google Scholar]
- 62.Qiao HP, Gao WS, Huo JX, Yang ZS. Long Non-coding RNA GAS5 Functions as a Tumor Suppressor in Renal Cell Carcinoma. Asian Pacific Journal of Cancer Prevention. 2013;14:1077–82. doi: 10.7314/apjcp.2013.14.2.1077. [DOI] [PubMed] [Google Scholar]
- 63.Wang M, Huang T, Luo G, Huang C, Xiao XY, Wang L, Jiang GS, Zeng FQ. Long non-coding RNA MEG3 induces renal cell carcinoma cells apoptosis by activating the mitochondrial pathway. J Huazhong Univ Sci Technolog Med Sci. 2015;35:541–5. doi: 10.1007/s11596-015-1467-5. [DOI] [PubMed] [Google Scholar]
- 64.Liu Y, Qian J, Li X, Chen W, Xu A, Zhao K, Hua Y, Huang Z, Zhang J, Liang C, Su S, Li P, Shao P, et al. Long non-coding RNA BX357664 regulates cell proliferation and epithelial-to-mesenchymal transition via inhibition of TGF-β1/p38/HSP27 signaling in renal cell carcinoma. Oncotarget. 2016;7:81410–81422. doi: 10.18632/oncotarget.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su H, Sun T, Wang H, Shi G, Zhang H, Sun F, Ye D. Decreased TCL6 expression is associated with poor prognosis in patients with clear cell renal cell carcinoma. Oncotarget. 2017;8:5789–5799. doi: 10.18632/oncotarget.11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao Y, Xu R, Xu X, Zhou Y, Cui L, He X. Downregulation of lncRNA CASC2 by microRNA-21 increases the proliferation and migration of renal cell carcinoma cells. Mol Med Rep. 2016;14:1019–25. doi: 10.3892/mmr.2016.5337. [DOI] [PubMed] [Google Scholar]
- 67.Xue S, Li QW, Che JP, Guo Y, Yang FQ, Zheng JH. Decreased expression of long non-coding RNA NBAT-1 is associated with poor prognosis in patients with clear cell renal cell carcinoma. Int J Clin Exp Pathol. 2015;8:3765–74. [PMC free article] [PubMed] [Google Scholar]
- 68.Ellinger J, Alam J, Rothenburg J, Deng M, Schmidt D, Syring I, Miersch H, Perner S, Müller SC. The long non-coding RNA lnc-ZNF180-2 is a prognostic biomarker in patients with clear cell renal cell carcinoma. Am J Cancer Res. 2015;5:2799–807. [PMC free article] [PubMed] [Google Scholar]
- 69.Duan W, Du L, Jiang X, Wang R, Yan S, Xie Y, Yan K, Wang Q, Wang L, Zhang X, Pan H, Yang Y, Wang C. Identification of a serum circulating lncRNA panel for the diagnosis and recurrence prediction of bladder cancer. Oncotarget. 2016;7:78850–78858. doi: 10.18632/oncotarget.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng C, Hao H, Chen L, Shao J. Long noncoding RNAs as novel serum biomarkers for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Clin Transl Oncol. 2017 Feb;10 doi: 10.1007/s12094-017-1626-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 71.Yang JP, Yang XJ, Xiao L, Wang Y. Long noncoding RNA PVT1 as a novel serum biomarker for detection of cervical cancer. Eur Rev Med Pharmacol Sci. 2016;20:3980–3986. [PubMed] [Google Scholar]
- 72.Jin C, Shi W, Wang F, Shen X, Qi J, Cong H, Yuan J, Shi L, Zhu B, Luo X, Zhang Y, Ju S. Long non-coding RNA HULC as a novel serum biomarker for diagnosis and prognosis prediction of gastric cancer. Oncotarget. 2016;7:51763–51772. doi: 10.18632/oncotarget.10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang C, Yu J, Han Y, Li L, Li J, Li T, Qi P. Long non-coding RNAs LOC285194, RP11-462C24.1 and Nbla12061 in serum provide a new approach for distinguishing patients with colorectal cancer from healthy controls. Oncotarget. 2016;7:70769–70778. doi: 10.18632/oncotarget.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu T, Zhang X, Gao S, Jing F, Yang Y, Du L, Zheng G, Li P, Li C, Wang C. Exosomal long noncoding RNA CRNDE-h as a novel serum-based biomarker for diagnosis and prognosis of colorectal cancer. Oncotarget. 2016;7:85551–85563. doi: 10.18632/oncotarget.13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miao Y, Fan R, Chen L, Qian H. Clinical Significance of Long Non-coding RNA MALAT1 Expression in Tissue and Serum of Breast Cancer. Ann Clin Lab Sci. 2016;46:418–24. [PubMed] [Google Scholar]
- 76.Wu Y, Wang YQ, Weng WW, Zhang QY, Yang XQ, Gan HL, Yang YS, Zhang PP, Sun MH, Xu MD, Wang CF. A serum circulating long non-coding RNA signature can discriminate between patients with clear cell renal cell carcinoma and healthy controls. Oncogenesis. 2016;5:e192. doi: 10.1038/oncsis.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sakurai K, Reon BJ, Anaya J, Dutta A. The lncRNA DRAIC/PCAT29 Locus Constitutes a Tumor-Suppressive Nexus. Mol Cancer Res. 2015;13:828–838. doi: 10.1158/1541-7786.MCR-15-0016-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–23. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee M, Kim HJ, Kim SW, Park SA, Chun KH, Cho NH, Song YS, Kim YT. The long non-coding RNA HOTAIR increases tumour growth and invasion in cervical cancer by targeting the Notch pathway. Oncotarget. 2016;7:44558–44571. doi: 10.18632/oncotarget.10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bhan A, Mandal SS. LncRNA HOTAIR: a master regulator of chromatin dynamics and cancer. Biochim Biophys Acta. 2015;1856:151–64. doi: 10.1016/j.bbcan.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, Shi Y, Segal E, Chang HY. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–93. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma XY, Wang JH, Wang JL, Ma CX, Wang XC, Liu FS. Malat1 as an evolutionarily conserved lncRNA plays a positive role in regulating proliferation and maintaining the undifferentiated status of early-stage hematopoietic cells. BMC Genomics. 2015;16:676. doi: 10.1186/s12864-015-1881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Asseldonk M, Schepens M, de Bruijn D, Janssen B, Merkx G, Geurts van Kessel A. Construction of a 350-kb sequence-ready 11q13 cosmid contig encompassing the markers D11S4933 and D11S546: mapping of 11 genes and 3 tumor-associated translocation breakpoints. Genomics. 2000;66:35–42. doi: 10.1006/geno.2000.6194. [DOI] [PubMed] [Google Scholar]
- 84.Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, Blencowe BJ, Prasanth SG, Prasanth KV. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–38. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xiao H, Tang K, Liu P, Chen K, Hu J, Zeng J, Xiao W, Yu G, Yao W, Zhou H, Li H, Pan Y, Li A, et al. LncRNA MALAT1 functions as a competing endogenous RNA to regulate ZEB2 expression by sponging miR-200s in clear cell. Oncotarget. 2015;6:38005–15. doi: 10.18632/oncotarget.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cai X, Cullen BR. The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA. 2007;13:313–6. doi: 10.1261/rna.351707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu Z, Yang F, Wei D, Liu B, Chen C, Bao Y, Wu Z, Wu D, Tan H, Li J, Wang J, Liu J, Sun S, et al. Long non-coding RNA-SRLR elicits intrinsic sorafenib resistance via evoking IL-6/STAT3 axis in renal cell carcinoma. Oncogene. doi: 10.1038/onc.2016.356. 2016 Nov 14. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 88.Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y, Chen W, Liu F, Sun W, Li XF, Wang X, Wang Y, Xu ZY, et al. Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA. Cancer Cell. 2016;29:653–68. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 89.Qu L, Wu Z, Li Y, Xu Z, Liu B, Liu F, Bao Y, Wu D, Liu J, Wang A, Chu X, Sun Y, Chen C, et al. A feed-forward loop between lncARSR and YAP activity promotes the expansion of renal tumour-initiating cells. Nat Commun. 2016;7:12692. doi: 10.1038/ncomms12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhai W, Sun Y, Jiang M, Wang M, Gasiewicz TA, Zheng J, Chang C. Differential regulation of LncRNA-SARCC suppresses VHL-mutant RCC cell proliferation yet promotes VHL-normal RCC cell proliferation via modulating androgen receptor/HIF-2α/C-MYC axis under hypoxia. Oncogene. 2016;35:4866–80. doi: 10.1038/onc.2016.19. [DOI] [PubMed] [Google Scholar]
- 91.Shi X, Xu Y, Zhang C, Feng L, Sun Z, Han J, Su F, Zhang Y, Li C, Li X. Subpathway-LNCE: Identify dysfunctional subpathways competitively regulated by lncRNAs through integrating lncRNA-mRNA expression profile and pathway topologies. Oncotarget. 2016;7:69857–69870. doi: 10.18632/oncotarget.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]


