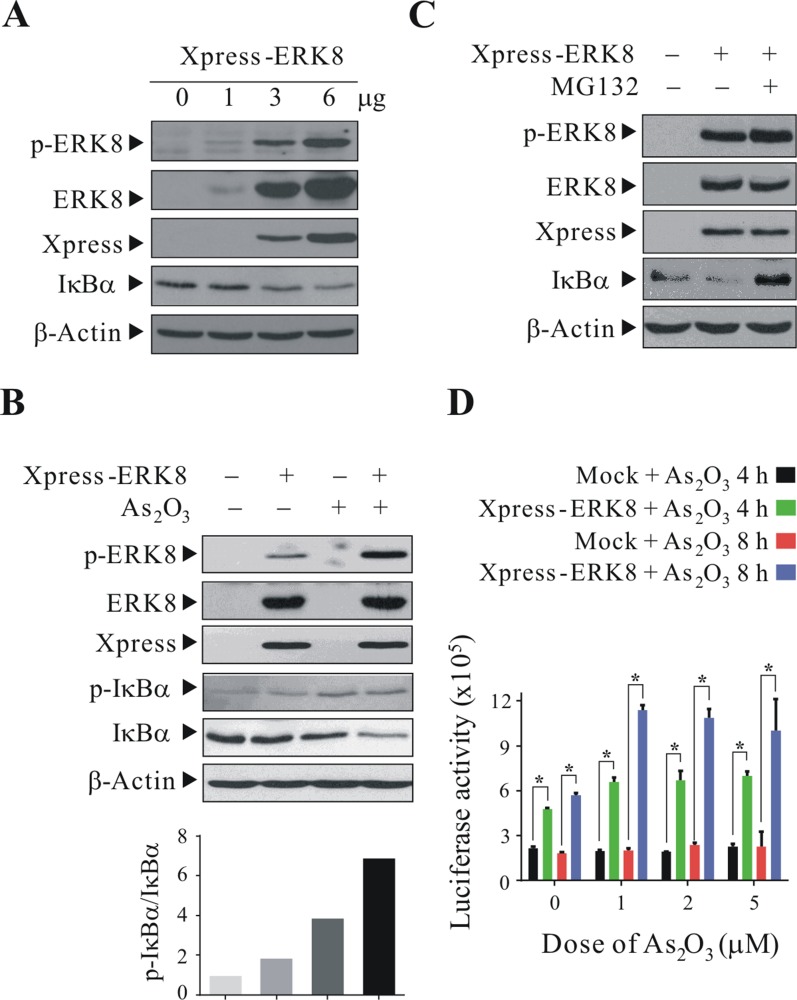
Figure 3. ERK8 promotes the phosphorylation and degradation of IκBα.
HEK293T cells were transfected with Xpress-ERK8 expression plasmid. At 24 h post-transfection, they were cultured in serum-free medium for 24 h, after which they were exposed to 5 μM As2O3 for 4 h. (A) cells transfected with 1 to 6 μg Xpress-ERK8 expression plasmid; (B) cells transfected with 6 μg Xpress-ERK8 expression plasmid; (C) cells transfected with 6 μg Xpress-ERK8 expression plasmid, and prior to As2O3 treatment, cells were first pretreated with 10 μM MG132 for 4 h. Cells were lysed, and protein extracts were subjected to SDS-PAGE followed by immunobloting using antibodies against p-ERK8 and p-IκBα (Ser32/Ser36). After development, the membranes were stripped and reprobed with regular antibodies against ERK8, Xpress, IκBα, and β-actin to monitor the total level of ERK8, IκBα and loading difference, respectively. The intensities of the bands of p-IκBα and IκBα in (B) were quantified and expressed as relative ratios, setting 1 for control. (D) NF-κB luciferase assay using NIH/3T3 cells treated with As2O3. NIH/3T3 cells seeded at 6 cm dish were transient transfected with ERK8 expressing plasmid (6 μg) along with the 5× kB luciferase reporter plasmid (6 μg) and the Renilla luciferase reporter plasmid (150 ng). At 24 h post-transfection, they were seeded into 24 well-plate at 1 × 105 cells/well. After 24 h, they were sham-exposed or exposed to 1, 2, or 5 μM As2O3 for additional 4 and 8 h. NF-κB-luciferase activity was measured and normalized against Renilla luciferase activity as described in “Materials and methods”. *A significant difference of P < 0.05. The data are representative of three independent experiments.