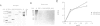Abstract
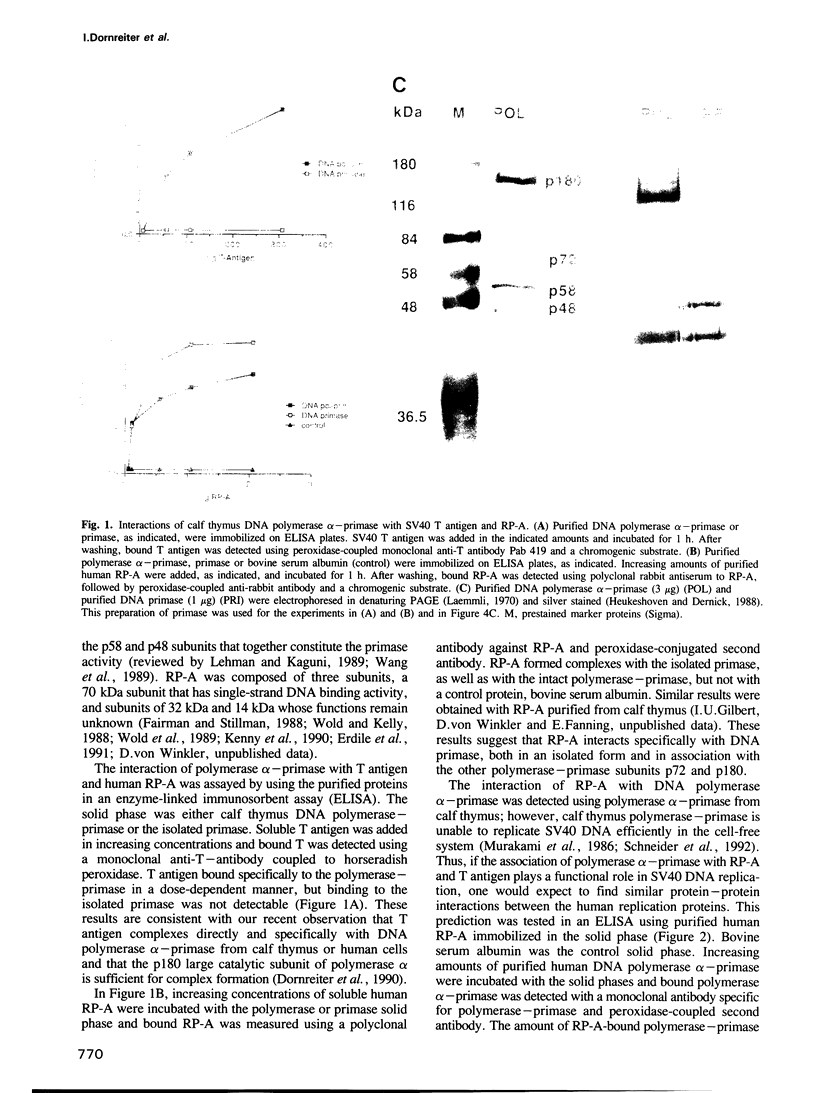
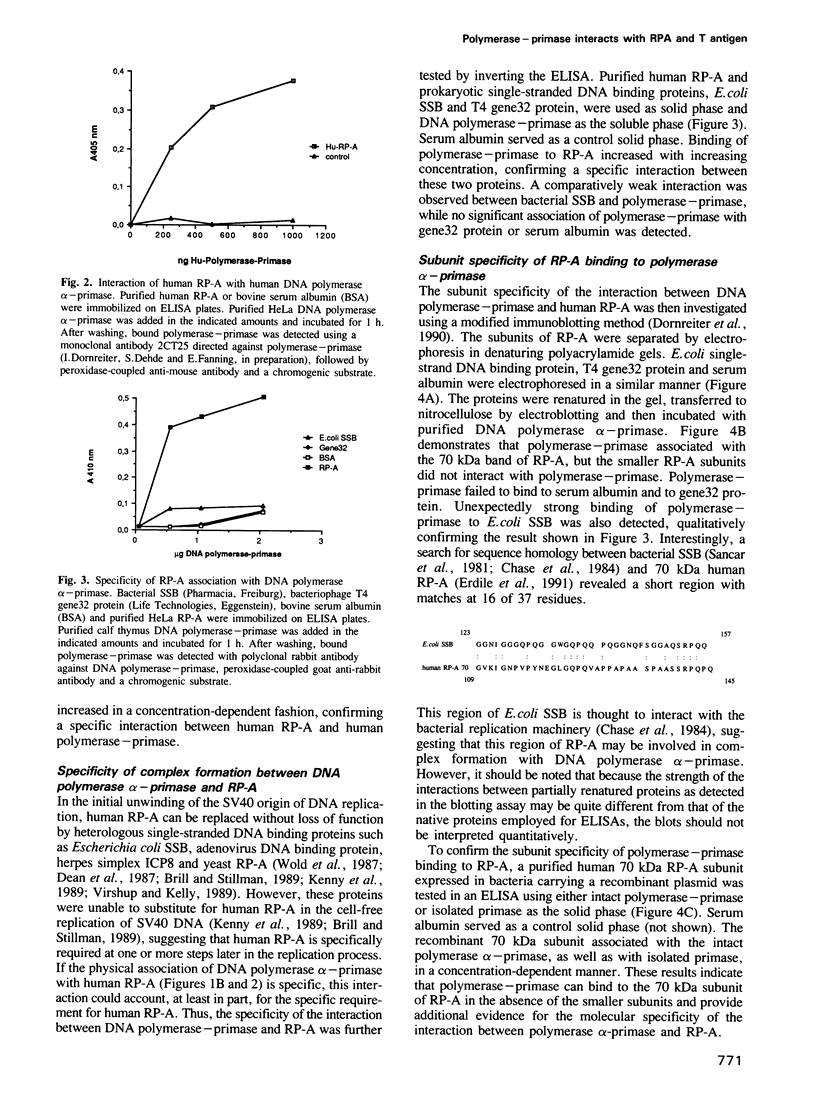
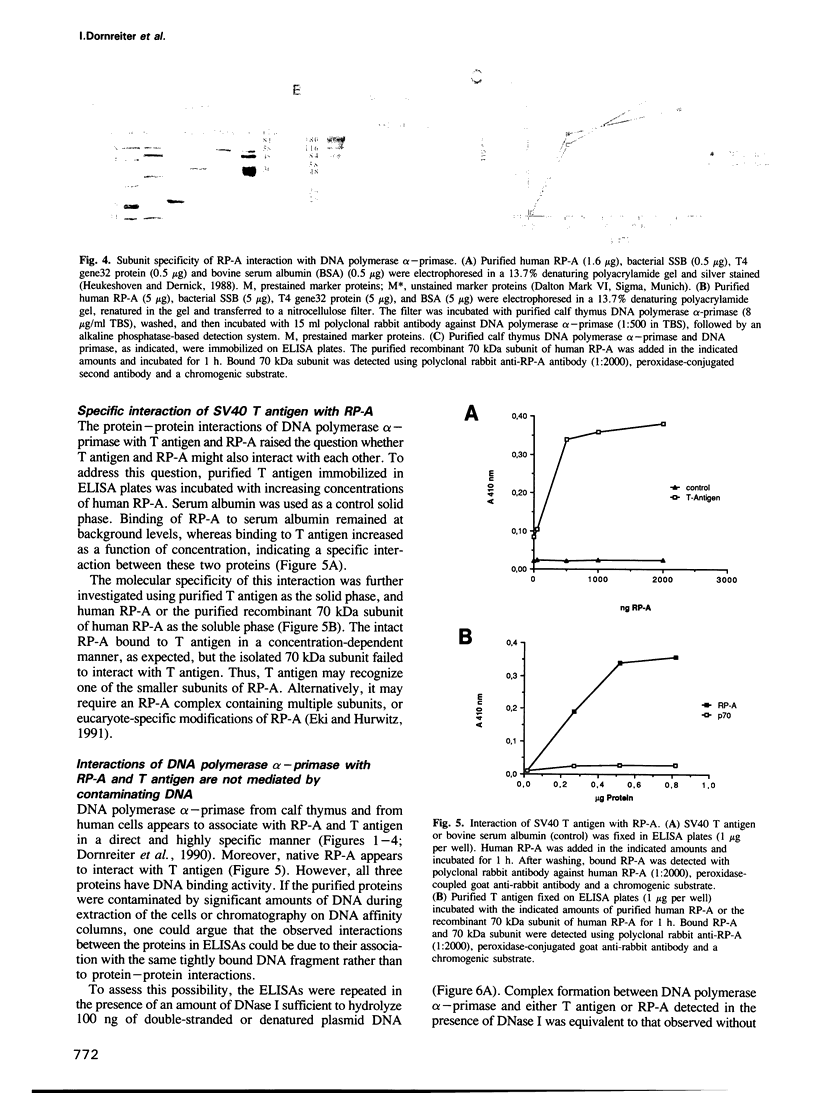
The purified human single-stranded DNA binding protein, replication protein A (RP-A), forms specific complexes with purified SV40 large T antigen and with purified DNA polymerase alpha-primase, as shown by ELISA and a modified immunoblotting technique. RP-A associated efficiently with the isolated primase, as well as with intact polymerase alpha-primase. The 70 kDa subunit of RP-A was sufficient for association with polymerase alpha-primase. Purified SV40 large T antigen bound to intact RP-A and to polymerase-primase, but not to any of the separated subunits of RP-A or to the isolated primase. These results suggest that the specific protein-protein interactions between RP-A, polymerase-primase and T antigen may play a role in the initiating of SV40 DNA replication.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfano C., McMacken R. Heat shock protein-mediated disassembly of nucleoprotein structures is required for the initiation of bacteriophage lambda DNA replication. J Biol Chem. 1989 Jun 25;264(18):10709–10718. [PubMed] [Google Scholar]
- Alfano C., McMacken R. Ordered assembly of nucleoprotein structures at the bacteriophage lambda replication origin during the initiation of DNA replication. J Biol Chem. 1989 Jun 25;264(18):10699–10708. [PubMed] [Google Scholar]
- Borowiec J. A., Dean F. B., Bullock P. A., Hurwitz J. Binding and unwinding--how T antigen engages the SV40 origin of DNA replication. Cell. 1990 Jan 26;60(2):181–184. doi: 10.1016/0092-8674(90)90730-3. [DOI] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. A model for initiation at origins of DNA replication. Cell. 1988 Sep 23;54(7):915–918. doi: 10.1016/0092-8674(88)90102-x. [DOI] [PubMed] [Google Scholar]
- Brill S. J., Stillman B. Yeast replication factor-A functions in the unwinding of the SV40 origin of DNA replication. Nature. 1989 Nov 2;342(6245):92–95. doi: 10.1038/342092a0. [DOI] [PubMed] [Google Scholar]
- Bullock P. A., Seo Y. S., Hurwitz J. Initiation of simian virus 40 DNA replication in vitro: pulse-chase experiments identify the first labeled species as topologically unwound. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3944–3948. doi: 10.1073/pnas.86.11.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- Chase J. W., L'Italien J. J., Murphy J. B., Spicer E. K., Williams K. R. Characterization of the Escherichia coli SSB-113 mutant single-stranded DNA-binding protein. Cloning of the gene, DNA and protein sequence analysis, high pressure liquid chromatography peptide mapping, and DNA-binding studies. J Biol Chem. 1984 Jan 25;259(2):805–814. [PubMed] [Google Scholar]
- Collins K. L., Kelly T. J. Effects of T antigen and replication protein A on the initiation of DNA synthesis by DNA polymerase alpha-primase. Mol Cell Biol. 1991 Apr;11(4):2108–2115. doi: 10.1128/mcb.11.4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean F. B., Bullock P., Murakami Y., Wobbe C. R., Weissbach L., Hurwitz J. Simian virus 40 (SV40) DNA replication: SV40 large T antigen unwinds DNA containing the SV40 origin of replication. Proc Natl Acad Sci U S A. 1987 Jan;84(1):16–20. doi: 10.1073/pnas.84.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson M., McMacken R., Echols H. Specialized nucleoprotein structures at the origin of replication of bacteriophage lambda. Protein association and disassociation reactions responsible for localized initiation of replication. J Biol Chem. 1989 Jun 25;264(18):10719–10725. [PubMed] [Google Scholar]
- Dornreiter I., Höss A., Arthur A. K., Fanning E. SV40 T antigen binds directly to the large subunit of purified DNA polymerase alpha. EMBO J. 1990 Oct;9(10):3329–3336. doi: 10.1002/j.1460-2075.1990.tb07533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn S. D. Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Anal Biochem. 1986 Aug 15;157(1):144–153. doi: 10.1016/0003-2697(86)90207-1. [DOI] [PubMed] [Google Scholar]
- Eki T., Hurwitz J. Influence of poly(ADP-ribose) polymerase on the enzymatic synthesis of SV40 DNA. J Biol Chem. 1991 Feb 15;266(5):3087–3100. [PubMed] [Google Scholar]
- Erdile L. F., Heyer W. D., Kolodner R., Kelly T. J. Characterization of a cDNA encoding the 70-kDa single-stranded DNA-binding subunit of human replication protein A and the role of the protein in DNA replication. J Biol Chem. 1991 Jun 25;266(18):12090–12098. [PubMed] [Google Scholar]
- Erdile L. F., Wold M. S., Kelly T. J. The primary structure of the 32-kDa subunit of human replication protein A. J Biol Chem. 1990 Feb 25;265(6):3177–3182. [PubMed] [Google Scholar]
- Fairman M. P., Stillman B. Cellular factors required for multiple stages of SV40 DNA replication in vitro. EMBO J. 1988 Apr;7(4):1211–1218. doi: 10.1002/j.1460-2075.1988.tb02933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon J. V., Lane D. P. Interactions between SV40 T antigen and DNA polymerase alpha. New Biol. 1990 Jan;2(1):84–92. [PubMed] [Google Scholar]
- Gannon J. V., Lane D. P. p53 and DNA polymerase alpha compete for binding to SV40 T antigen. Nature. 1987 Oct 1;329(6138):456–458. doi: 10.1038/329456a0. [DOI] [PubMed] [Google Scholar]
- Gill S. C., von Hippel P. H. Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem. 1989 Nov 1;182(2):319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. T., Russell W. C. Recognition mechanisms in the synthesis of animal virus DNA. Biochem J. 1989 Feb 15;258(1):3–16. doi: 10.1042/bj2580003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heukeshoven J., Dernick R. Improved silver staining procedure for fast staining in PhastSystem Development Unit. I. Staining of sodium dodecyl sulfate gels. Electrophoresis. 1988 Jan;9(1):28–32. doi: 10.1002/elps.1150090106. [DOI] [PubMed] [Google Scholar]
- Hurwitz J., Dean F. B., Kwong A. D., Lee S. H. The in vitro replication of DNA containing the SV40 origin. J Biol Chem. 1990 Oct 25;265(30):18043–18046. [PubMed] [Google Scholar]
- Höss A., Moarefi I., Scheidtmann K. H., Cisek L. J., Corden J. L., Dornreiter I., Arthur A. K., Fanning E. Altered phosphorylation pattern of simian virus 40 T antigen expressed in insect cells by using a baculovirus vector. J Virol. 1990 Oct;64(10):4799–4807. doi: 10.1128/jvi.64.10.4799-4807.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimi Y., Claude A., Bullock P., Hurwitz J. Complete enzymatic synthesis of DNA containing the SV40 origin of replication. J Biol Chem. 1988 Dec 25;263(36):19723–19733. [PubMed] [Google Scholar]
- Kenny M. K., Lee S. H., Hurwitz J. Multiple functions of human single-stranded-DNA binding protein in simian virus 40 DNA replication: single-strand stabilization and stimulation of DNA polymerases alpha and delta. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9757–9761. doi: 10.1073/pnas.86.24.9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny M. K., Schlegel U., Furneaux H., Hurwitz J. The role of human single-stranded DNA binding protein and its individual subunits in simian virus 40 DNA replication. J Biol Chem. 1990 May 5;265(13):7693–7700. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanford R. E. Expression of simian virus 40 T antigen in insect cells using a baculovirus expression vector. Virology. 1988 Nov;167(1):72–81. doi: 10.1016/0042-6822(88)90055-4. [DOI] [PubMed] [Google Scholar]
- Lehman I. R., Kaguni L. S. DNA polymerase alpha. J Biol Chem. 1989 Mar 15;264(8):4265–4268. [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo I. A., Hough P. V., Wall J. S., Dodson M., Dean F. B., Hurwitz J. ATP-dependent assembly of double hexamers of SV40 T antigen at the viral origin of DNA replication. Nature. 1989 Apr 20;338(6217):658–662. doi: 10.1038/338658a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto T., Eki T., Hurwitz J. Studies on the initiation and elongation reactions in the simian virus 40 DNA replication system. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9712–9716. doi: 10.1073/pnas.87.24.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskimins W. K., Roberts M. P., McClelland A., Ruddle F. H. Use of a protein-blotting procedure and a specific DNA probe to identify nuclear proteins that recognize the promoter region of the transferrin receptor gene. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6741–6744. doi: 10.1073/pnas.82.20.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y., Wobbe C. R., Weissbach L., Dean F. B., Hurwitz J. Role of DNA polymerase alpha and DNA primase in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1986 May;83(9):2869–2873. doi: 10.1073/pnas.83.9.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasheuer H. P., Grosse F. Immunoaffinity-purified DNA polymerase alpha displays novel properties. Biochemistry. 1987 Dec 15;26(25):8458–8466. doi: 10.1021/bi00399a064. [DOI] [PubMed] [Google Scholar]
- Prives C. The replication functions of SV40 T antigen are regulated by phosphorylation. Cell. 1990 Jun 1;61(5):735–738. doi: 10.1016/0092-8674(90)90179-i. [DOI] [PubMed] [Google Scholar]
- Sancar A., Williams K. R., Chase J. W., Rupp W. D. Sequences of the ssb gene and protein. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4274–4278. doi: 10.1073/pnas.78.7.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale S. T., Tjian R. T-antigen-DNA polymerase alpha complex implicated in simian virus 40 DNA replication. Mol Cell Biol. 1986 Nov;6(11):4077–4087. doi: 10.1128/mcb.6.11.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H., Dröge P., Knippers R. DNA helicase activity of SV40 large tumor antigen. EMBO J. 1986 Aug;5(8):1939–1944. doi: 10.1002/j.1460-2075.1986.tb04447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Takada-Takayama R., Tada S., Hanaoka F., Ui M. Peptide mapping of the four subunits of the mouse DNA polymerase alpha-primase complex. Biochem Biophys Res Commun. 1990 Jul 31;170(2):589–595. doi: 10.1016/0006-291x(90)92132-j. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Melendy T., Stillman B. Sequential initiation of lagging and leading strand synthesis by two different polymerase complexes at the SV40 DNA replication origin. Nature. 1990 Aug 9;346(6284):534–539. doi: 10.1038/346534a0. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Stillman B. Multiple replication factors augment DNA synthesis by the two eukaryotic DNA polymerases, alpha and delta. EMBO J. 1989 Dec 1;8(12):3883–3889. doi: 10.1002/j.1460-2075.1989.tb08567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Stillman B. Replication factors required for SV40 DNA replication in vitro. I. DNA structure-specific recognition of a primer-template junction by eukaryotic DNA polymerases and their accessory proteins. J Biol Chem. 1991 Jan 25;266(3):1950–1960. [PubMed] [Google Scholar]
- Tsurimoto T., Stillman B. Replication factors required for SV40 DNA replication in vitro. II. Switching of DNA polymerase alpha and delta during initiation of leading and lagging strand synthesis. J Biol Chem. 1991 Jan 25;266(3):1961–1968. [PubMed] [Google Scholar]
- Virshup D. M., Kelly T. J. Purification of replication protein C, a cellular protein involved in the initial stages of simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1989 May;86(10):3584–3588. doi: 10.1073/pnas.86.10.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. S., Wong S. W., Korn D. Human DNA polymerase alpha: predicted functional domains and relationships with viral DNA polymerases. FASEB J. 1989 Jan;3(1):14–21. doi: 10.1096/fasebj.3.1.2642867. [DOI] [PubMed] [Google Scholar]
- Weinberg D. H., Collins K. L., Simancek P., Russo A., Wold M. S., Virshup D. M., Kelly T. J. Reconstitution of simian virus 40 DNA replication with purified proteins. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8692–8696. doi: 10.1073/pnas.87.22.8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., Kelly T. Purification and characterization of replication protein A, a cellular protein required for in vitro replication of simian virus 40 DNA. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2523–2527. doi: 10.1073/pnas.85.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., Li J. J., Kelly T. J. Initiation of simian virus 40 DNA replication in vitro: large-tumor-antigen- and origin-dependent unwinding of the template. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3643–3647. doi: 10.1073/pnas.84.11.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold M. S., Weinberg D. H., Virshup D. M., Li J. J., Kelly T. J. Identification of cellular proteins required for simian virus 40 DNA replication. J Biol Chem. 1989 Feb 15;264(5):2801–2809. [PubMed] [Google Scholar]
- Yoshimura N., Nakai I., Ohmori Y., Aikawa I., Fukuda M., Yasumura T., Matsui S., Hamashima T., Oka T. Effect of cyclosporine on the endocrine and exocrine pancreas in kidney transplant recipients. Am J Kidney Dis. 1988 Jul;12(1):11–17. doi: 10.1016/s0272-6386(88)80065-9. [DOI] [PubMed] [Google Scholar]