Table 2.
SAR of the merged tricyclic indole derivatives for binding to Mcl-1 and Bcl-xL
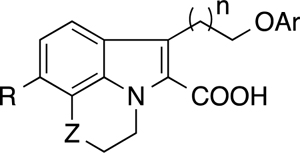
| ||||||||
|---|---|---|---|---|---|---|---|---|
| compd | Z | n | R | Ar | Mcl-1 |
Ki (µM) Bcl-xL |
Bcl-2 | |
| 16 | S | 1 | H | 1-naphthyl | 3.0 | 9.0 | ||
| 17 | CH2 | 1 | H | 1-naphthyl | 4.0 | >20 | ||
| 18 | 0 | 1 | H | 1-naphthyl | 1.6 | 12.7 | ||
| 19 | S | 2 | H | 1-naphthyl | 0.12 | >20 | 3.1 | |
| 20 | CH2 | 2 | H | 1-naphthyl | 0.31 | >20 | 7.3 | |
| 21 | 0 | 2 | H | 1-naphthyl | 0.11 | 1.9 | 5.7 | |
| 22 | S02 | 2 | H | 1-naphthyl | 0.088 | >20 | ||
| 23 | SCH2 | 2 | H | 1-naphthyl | 0.17 | 7.4 | 9.9 | |
| 24 | SOCH2 | 2 | H | 1-naphthyl | 0.074 | >20 | 16.0 | |
| 25 | S02CH2 | 2 | H | 1-naphthyl | 0.061 | >20 | 11.7 | |
| 26 | S | 2 | H | 1 -(5,6,7,8-tetrahy dronaphthyl) | 0.150 | 3.0 | 3.7 | |
| 27 | S | 2 | H | 1-(4-Cl-naphthyl) | 0.200 | 4.7 | 1.1 | |
| 28 | S | 2 | H | 2-(5,6,7,8-tetrahydronaphthyl) | 0.410 | >20 | 4.4 | |
| 29 | S | 2 | H | 3-Me4-a-phenyl | 0.210 | >20 | 6.3 | |
| 30 | S | 2 | H | 3,5-di-Me4-Cl-phenyl-phenyl | 0.071 | >20 | 2.3 | |
| 31 | CH2 | 2 | H | 3,5-di-Me4-Cl-phenyl-phenyl | 0.110 | 7.8 | ||
| 32 | 0 | 2 | H | 3,5-di-Me4-Cl-phenyl-phenyl | 0.065 | |||
| 33 | SCH2 | 2 | H | 3,5-di-Me4-Cl-phenyl-phenyl | 0.040 | >20 | 3.7 | |
| 34 | CH2 | 2 | CI | 3,5-di-Me4-Cl-phenyl-phenyl | 0.003 | 5.2 | 0.77 | |
| 35 | 0 | 2 | CI | 3,5-di-Me4-Cl-phenyl-phenyl | 0.009 | >20 | 1.3 | |
