Figure 4.
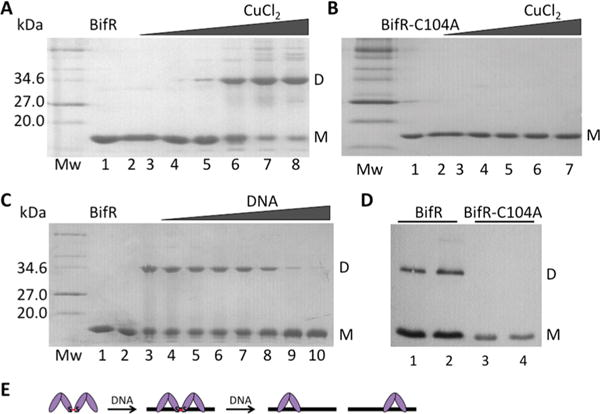
BifR oxidation by copper. In panels (A–C), left lane is protein marker (Mw; in kDa), lane 1 is air-oxidized protein, and lane 2 is reduced protein. (A) BifR with increasing concentration of CuCl2 (lanes 3–8; 5 μM to 2.5 mM). (B) BifR-C104A with increasing concentration of CuCl2 (lanes 3–7; 5 μM to 1 mM). (C) Lane 3, BifR oxidized with 500 μM CuCl2; lanes 4–10, BifR with increasing concentration of operator DNA (10–60 μM) followed by addition of 500 μM CuCl2. (D) Western blot of lysate from E. coli BL21(DE3)-pLysS using antibody to His6-tagged BifR (lanes 1 and 2) and BifR-C104A (lanes 3 and 4); lanes 2 and 4 correspond to cultures grown in the presence of 1 mM H2O2 for 30 min. Monomer (M) migrates with a Mw ≈ 18 kDa and dimer (D) migrates with a Mw ≈ 36 kDa; monomer and dimer is indicated to the right of each image. (E) Interpretation of data from panel (C) in which absence of cross-linked species requires a stoichiometric excess of DNA.
