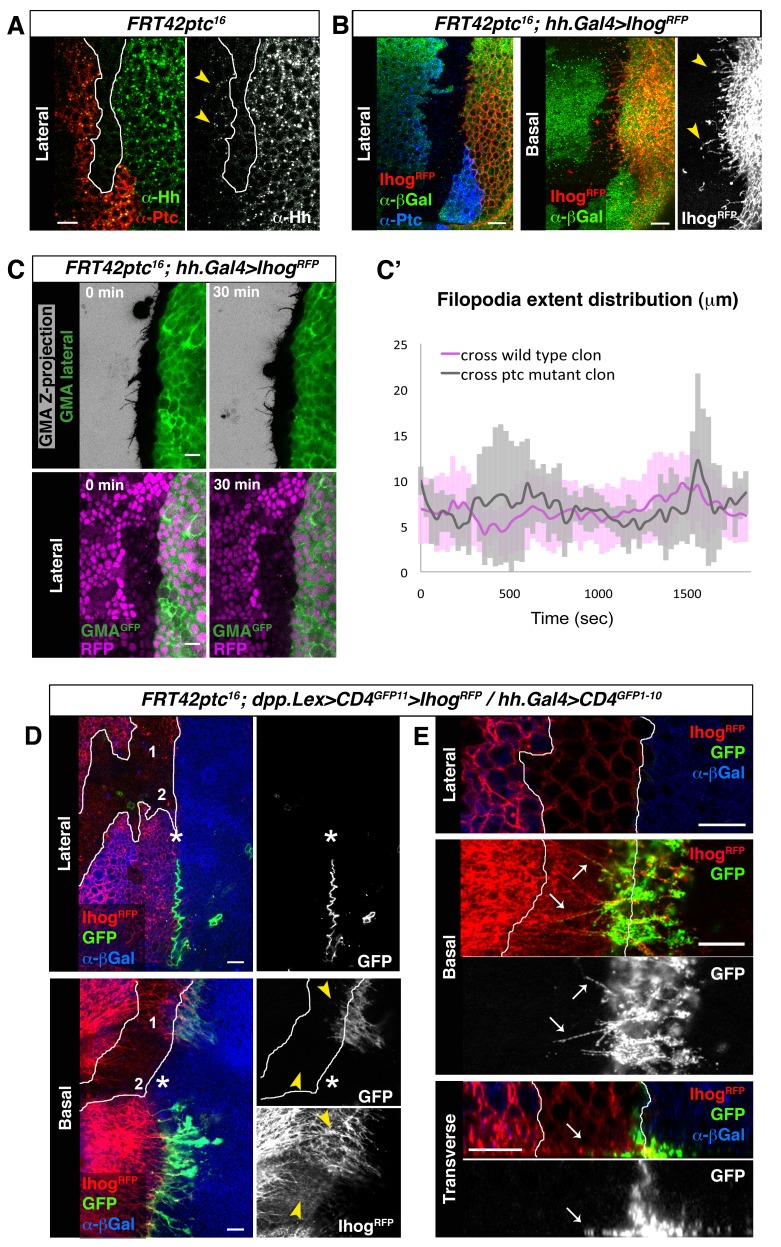
Figure 5. Cytonemes cross ptc−/− mutant clones at the A/P compartment border.
(A) A ptc16 null clone in the A compartment abutting the A/P compartment border of a wing disc co-labelled with α-Hh and α-Ptc antibodies. Note that both Ptc and Hh proteins are detected anterior to the clone in the A compartment (arrowheads). (B) P compartment cytonemes extend through a ptc−/− clone (arrowheads). Lateral and basal sections of a ptc−/− clone (absence of βGal) in the A compartment with Ihog-RFP expression in the P compartment to visualize cytonemes (FRT42D ptc16 / hh.Gal4, tub.Gal80ts>UAS.ihog-RFP wing disc after 24 hr at the restrictive temperature) co-labelled with α-βGal and α-Ptc antibodies. (C) First and last time frames from Video 6 displaying a lateral view of GMA-GFP signal to easily visualize cell perimeters together with a Z-projection of GMA-GFP where filopodia are shown (top panels) or with a lateral view of nuclear RFP to distinguish between wild-type (magenta nuclei) and ptc−/− mutant (absence of magenta nuclei) territories (bottom panels). Scale bars represent 10 μm. (C’) Graph showing extent distribution over time of GMA-GFP filopodia emanating from Hh-producing cells. Notice that there is no difference between filopodia crossing a wild-type (magenta) or a ptc−/− mutant (grey) clone territories. (D) A ptc16 clone induced in the A compartment abutting the compartment border in a dpp.LexA>LexAop.CD4-GFP11>LexAop.ihog-RFP / tub.Gal80ts, hh.Gal4>UAS.CD4-GFP1-10 wing disc after 24 hr at the restrictive temperature and labelled with α-βGal antibody to identify the mutant clone (absence of βGal). Note the GRASP signal is not visualized laterally (asterisk). Note also that basal cytonemes cross the ptc−/− clone in region 1 but not in region 2, and that the GRASP signal is restricted to region 1 and absent from region 2 (arrowheads). E) Another ptc−/− clone induced in a disc with the same genotype as in D showing the interaction between cytonemes from wild type cells anterior to the clone and cytonemes from the P compartment. Note the GRASP signal along basal cytonemes from wild type A compartment cells that traverse the ptc−/− clone (arrows). The data shown were consistent in at least three independent experiments with an average of 5–10 discs in each experiment. Bars, 10 µm.