Significance
Biologically available nitrogen (fixed N) limits the fertility of much of the ocean. Of the processes that remove fixed N from the ocean, conversion to N2 in coastal sediments appears to dominate. This work provides the strongest data-based support for the long-standing hypothesis of changes in N loss along the ocean margin due to the cyclic drowning and emergence of the continental shelves. The data also imply strong local coupling of N loss to N2 fixation, the dominant N input to the ocean, thus suggesting a stable oceanic fixed N reservoir over glacial cycles. Finally, this work points to glacial/interglacial oscillations in the biogeochemical fluxes at and near the ocean margins that would have influenced the evolution of coastal species.
Keywords: denitrification, nitrogen fixation, nitrogen isotopes, glacial cycles
Abstract
The continental shelves are the most biologically dynamic regions of the ocean, and they are extensive worldwide, especially in the western North Pacific. Their area has varied dramatically over the glacial/interglacial cycles of the last million years, but the effects of this variation on ocean biological and chemical processes remain poorly understood. Conversion of nitrate to N2 by denitrification in sediments accounts for half or more of the removal of biologically available nitrogen (“fixed N”) from the ocean. The emergence of continental shelves during ice ages and their flooding during interglacials have been hypothesized to drive changes in sedimentary denitrification. Denitrification leads to the occurrence of phosphorus-bearing, N-depleted surface waters, which encourages N2 fixation, the dominant N input to the ocean. An 860,000-y record of foraminifera shell-bound N isotopes from the South China Sea indicates that N2 fixation covaried with sea level. The N2 fixation changes are best explained as a response to changes in regional excess phosphorus supply due to sea level-driven variations in shallow sediment denitrification associated with the cyclic drowning and emergence of the continental shelves. This hypothesis is consistent with a glacial ocean that hosted globally lower rates of fixed N input and loss and a longer residence time for oceanic fixed N—a “sluggish” ocean N budget during ice ages. In addition, this work provides a clear sign of sea level-driven glacial/interglacial oscillations in biogeochemical fluxes at and near the ocean margins, with implications for coastal organisms and ecosystems.
Biological productivity in much of the ocean is limited by the supply of biologically available nitrogen (“fixed N”) (1). Biological processes are central to the input and output of fixed N to and from the ocean: N2 fixation by cyanobacteria in surface waters appears to dominate the input of N to the ocean, whereas the main sink is biological reduction to N2 (generalized here as “denitrification”) in sediments and in suboxic zones of the water column (2). Given this biologically determined input/output budget, the variation or constancy of the oceanic fixed N reservoir has broader implications for the potential of ocean life to regulate environmental conditions on a global scale. Because the “major nutrients” N and phosphorus (P) fuel the biological sequestration of CO2 in the deep ocean, changes in the oceanic fixed N reservoir have also been proposed as a driver of glacial/interglacial CO2 change (3, 4).
Sediment records show N isotopic evidence of reduced water column denitrification during the Last Glacial Maximum (LGM) and other cold phases of the glacial cycles relative to the current interglacial (the “Holocene”) and past warm time intervals (5, 6). “Benthic” denitrification (that which occurs in seafloor sediments) is equally as or more important than water column denitrification in the removal of N from the global ocean, and it has been hypothesized to decrease during glacials (times of high land ice volume) as well (7). This hypothesis is based on the generally rapid rate of denitrification in continental shelf sediments and on calculations that indicate the importance of shelf denitrification in the global ocean rate of denitrification (8). The continental shelves are characterized by high fluxes of organic matter to the sediments both because their shallow depth allows sinking matter to reach the bottom quickly and because the breakdown of organic matter in the shallow sediments returns nutrients immediately to the sunlit upper ocean. As a result, the nutrients supplied to the waters overlying the continental shelf drive multiple rapid cycles of productivity, sedimentation, and remineralization over its broad extent of shallow seafloor. During glacial maxima, the ∼120-m decline in sea level converted the continental shelves into coastal land, removing much of this environment as a site of oceanic N loss. The greater mean depth and steepness of the seaward continental slope should render the slope far less efficient at returning the nutrients released from the sediments to the upper ocean. Thus, upon sea level lowering, the coastal environment would be less favorable as an environment for both coastal productivity and benthic N loss. However, because the direct impact of benthic N loss on the N isotopes is typically nil or very weak (9, 10), there have been, as yet, no direct tests of this hypothesis.
Since the first studies of the ocean N budget, it has been recognized that a balance is required between inputs (dominantly N2 fixation) and losses (dominantly denitrification) on the timescale of the residence time of fixed N in the ocean [currently ∼3,000 y (2)], such that changes in N2 fixation should be coupled to, and thus provide evidence of, changes in denitrification. It has been argued that denitrification generates a selective advantage to N2 fixers by increasing the occurrence of phosphorus-bearing, N-depleted surface waters (i.e., excess phosphorus) (11). The resulting N2 fixation response may thus yield spatial and temporal coupling between denitrification and N2 fixation that balances the ocean’s N budget, for which there are multiple lines of evidence (12–14). However, it has been pointed out that N2 fixers have other sensitivities as well. In particular, both iron availability and temperature may be important constraints on N2 fixation (3, 15).
The South China Sea (SCS) repeats end of paragraph is a marginal sea characterized by a high ratio of shelf area to basin area (∼1.2). Deep SCS water has oceanographic characteristics similar to the western Pacific open ocean (16), with continuous exchange with the open western Pacific mainly through the Luzon Strait, which is ∼2,200 m deep, too deep for the exchange of thermocline and deeper water masses to have been affected by glacial/interglacial sea level change. The warm tropical surface waters of the SCS and the adjacent Asian dust sources and ocean margins appear to leave N2 fixation unconstrained by temperature or iron (17). The extensive East Asian and Sunda shelves host rapid sedimentary denitrification (8), which effectively removes fixed N and lowers the fixed nitrogen-to-phosphorus ratio (N/P) of the shallow water column in the region. These features suggest that the SCS may be prone to coupling between benthic denitrification and N2 fixation.
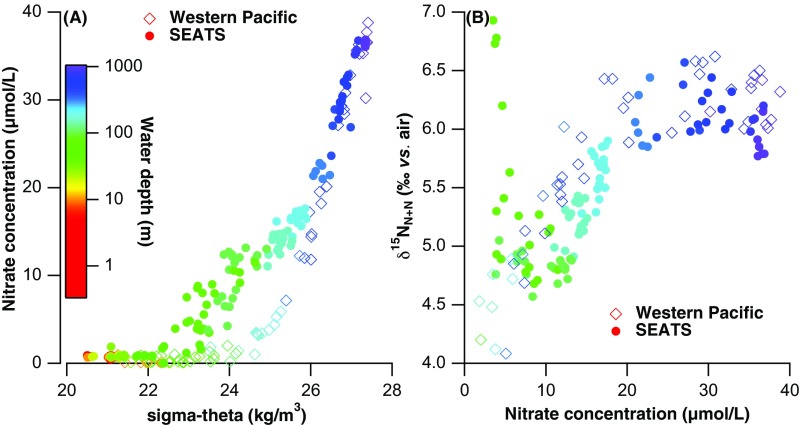
The nitrogen isotopes can be used to reconstruct past changes in N2 fixation in environments where the nitrogen isotopic signature of N2 fixation can be clearly observed in the thermocline. N2 fixation introduces N with a δ15N of ∼−1‰ versus atmospheric N2 (18), which is distinctly lower than the δ15N of oceanic nitrate (Fig. 1B). Mean ocean nitrate δ15N is elevated above that of the newly fixed N (9) because water column denitrification removes nitrate (NO3−) that is depleted in 15N (19). As a result, the remineralization of newly fixed N to nitrate causes regional lowering of nitrate δ15N underneath the surface waters in which N2 fixation occurs. This lowering is most intense in the shallow thermocline for two reasons. First, organic N is remineralized rapidly as it sinks, causing most of the sinking N and its isotopic signal of N2 fixation to be emplaced at shallow depths. Second, nitrate concentration decreases upward across the thermocline, helping the nitrate that derives from local or regional N2 fixation to represent a greater proportion of the total nitrate in the water. The lowering of nitrate δ15N by N2 fixation is perhaps most obvious today in the tropical and subtropical North Atlantic (20). However, a nitrate δ15N minimum in the shallow thermocline is also observed in the North Pacific (21), including the SCS (22) (Fig. 1C).
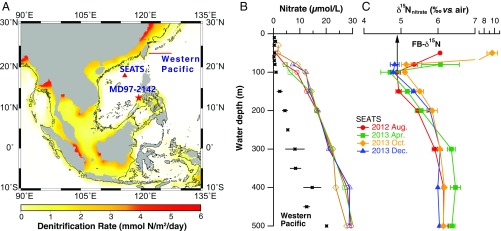
Fig. 1.
Core location and modern context for this study. (A) Topographic map showing the change in basin configuration around the SCS between interglacial sea level high stand and full glacial sea level low stand, assuming a 120-m lowering of the shore line on modern topography (63) (black contour; modern land area shown in gray). The locations of the coring site for core MD97-2142, the SEATS, and the hydrographic transect in the open western Pacific are shown with a star, triangle, and line, respectively. Colors depict model-simulated benthic denitrification rate (millimoles of N per square meter per day) (8). The 120-m ice age sea level lowering exposes almost all of the shallow shelf where benthic denitrification is rapid in the present day. (B and C) The depth profiles of the concentration and δ15N of nitrate plus nitrite in the upper 500 m at SEATS. The samples are collected from four cruises from 2012 summer to 2013 winter (indicated with different colors and symbols). The error bar at each depth indicates 1 SD associated with water collections from multiple casts during each cruise. The depth profile of the nitrate plus nitrite concentration in the open western Pacific is also shown for comparison (black squares). The remineralization of newly fixed N is taken as the dominant contributor to the subsurface nitrate δ15N minimum and also lowers the nitrate δ15N throughout the water column (22). The FB-δ15N of both G. ruber and O. universa measured at the surface sediment are 4.9‰ (black arrow), similar to the δ15N of the shallow thermocline nitrate being supplied to the photic zone.
The upward decline in nitrate δ15N in the SCS thermocline is not observed everywhere in the tropical and subtropical North Pacific; for example, it is not observed in the equatorial or subarctic North Pacific (23, 24). Thus, it must be a reflection of N2 fixation occurring in the western tropical/subtropical North Pacific. The shallow thermocline (i.e., the depth range of 100 m to 200 m) of the modern SCS has a much higher nitrate concentration (10 µM to 15 µM) than the same water depth or density level in the open subtropical North Pacific (<5 µM; Fig. 1B and Fig. S1). As a result, lateral exchange of the upper 200 m of the water column with the open western North Pacific has minimal capacity to change the δ15N of nitrate in this depth range of the SCS. Therefore, the upward decline in nitrate δ15N observed in the SCS thermocline (Fig. 1C) is probably mostly generated within the SCS.
Fig. S1.
Hydrographic evidence that the low nitrate δ15N in the shallow thermocline of the SCS derives from in situ remineralization of newly fixed N. (A) The nutrient concentration above 500 m is more elevated than in the open western Pacific on any given isopycnal. (B) The shallow SCS (between 100 m and 500 m) has a lower nitrate δ15N at a given nitrate concentration in comparison with the open western Pacific.
Nitrate from the shallow thermocline supplied by vertical mixing is the dominant N source to the tropical and subtropical surface ocean on an annual basis (25). Thus, the δ15N of the shallow thermocline nitrate is the dominant control on the δ15N of net biomass production in the surface ocean each year, which, in turn, sets the δ15N of the various species of planktonic foraminifera, the shells of which can be analyzed for the δ15N of their fossil-bound organic N (26). As a consequence, foraminifera-bound N has a lower δ15N in the modern SCS than in, for example, most of the equatorial Pacific (Fig. 2). Moreover, a higher rate of N2 fixation in the SCS would cause a further decline in foraminifera-bound δ15N (FB-δ15N), whereas slower N2 fixation would cause a δ15N rise.
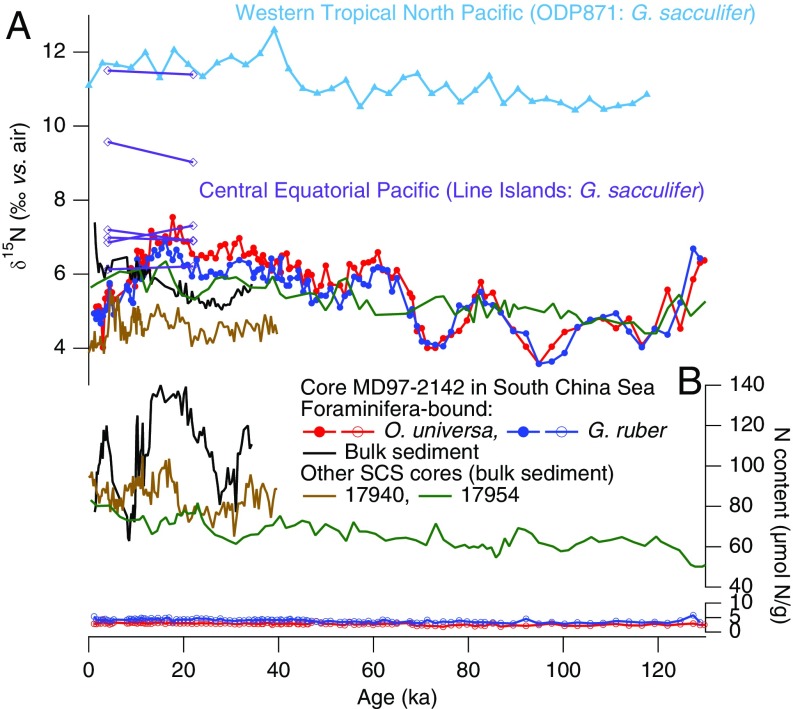
Fig. 2.
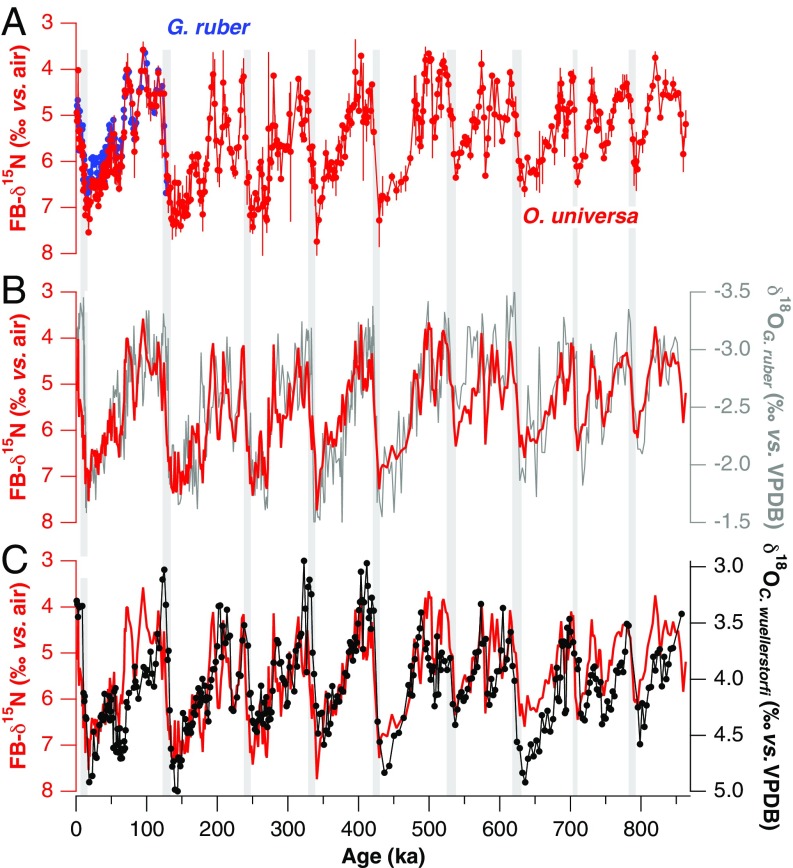
Compilation of the (A) δ15N and (B) N content of foraminifera-bound N and bulk sedimentary N records in the SCS and open Pacific. In SCS core MD97-2142, FB-δ15N (filled circles) and N content (open circles) are shown for the planktonic foraminifera species O. universa (red circles) and G. ruber (blue circles) and for bulk sedimentary N (black line). Previously published bulk sedimentary δ15N and N content from SCS cores 17940 and 17954 are also shown (green and brown lines) (27, 28). One open Pacific data set is a last ice age/Holocene FB-δ15N comparison in cores from along the Line Islands in the central equatorial Pacific (purple diamonds) (36). The latitudes of the five cores are 0.22°S, 1.27°N, 2.46°N, 2.97°N, 5.2°N, and 6.83°N. Each core corresponds to paired LGM and Holocene data, with the FB-δ15N from both the LGM and the Holocene increasing northward from the equator; from the assemblage of cores, no LGM-to-Holocene difference is observed. A second open Pacific data set is a record of FB-δ15N from the western tropical north Pacific [ODP Site 871 (5.56°N, 172.35°E); blue triangles]. This record shows no clear FB-δ15N decrease from the last ice age to the Holocene, in contrast to SCS FB-δ15N. The locations of the sediment cores are shown in Fig. S9.
Fig. S9.
The locations of the sediment cores in Fig. 2.
Results and Discussion
Here we report a record of FB-δ15N in the SCS over the last 860 ky, covering eight major glacial cycles (Methods). The sediment core is from site MD97-2142 on the slope off Palawan Island (Fig. 1A, 12°41′N, 119°27′E, water depth of 1,557 m, sedimentation rate of 10 cm/ky, age model shown in Fig. S2). The full record uses a single planktonic species, Orbulina universa. To test the generality of the O. universa FB-δ15N record, the FB-δ15N of Globigerinoides ruber was also analyzed over the last glacial cycle (back to ∼125 ka). FB-δ15N is expected to be similar for these two euphotic zone-dwelling species (26), and the data fit this expectation (Figs. 2 and 3). Slightly lower δ15N is observed for G. ruber than for O. universa during the last ice age, with an average offset of 0.39‰ for 20 ka to 60 ka compared with 0.25‰ for the entire overlapping period (Fig. 2). The same sense of divergence (with the δ15N of O. universa greater than that of G. ruber) is also observed in LGM samples from the Caribbean Sea (13), where it was tentatively interpreted to provide secondary support of the idea of reduced N2 fixation during the LGM (13); a similar explanation may apply in the SCS. In any case, the changes in interspecies FB-δ15N difference are minor relative to the FB-δ15N changes shared by the two species.
Fig. S2.
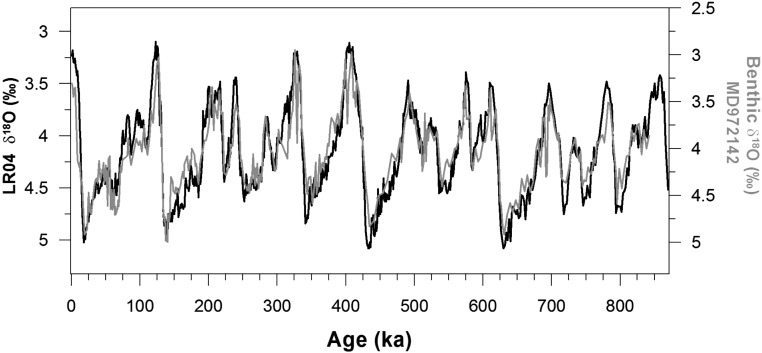
The benthic foraminifera calcite δ18O stratigraphy of MD972142. The age model of the core at MD972142 is reconstructed using correlation with LR04 benthic foraminifera oxygen isotope stack (74) and incorporates five published radiocarbon dates for the Holocene and Termination I (64). The benthic foraminifera δ18O record from MD972142 was analyzed on C. wuellerstorfi.
Fig. 3.
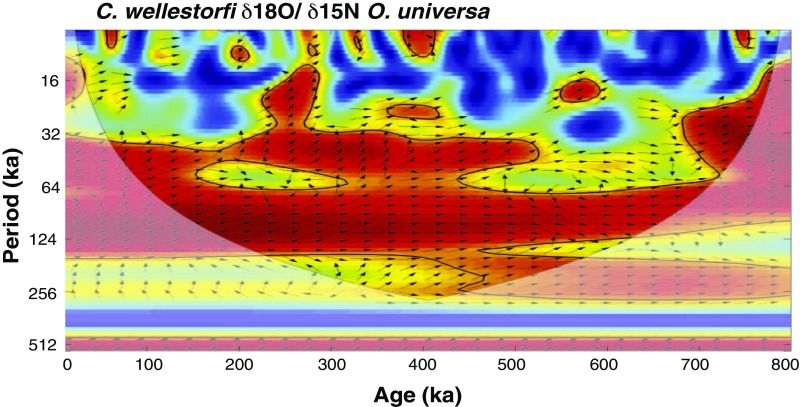
Records of planktonic FB-δ15N and planktonic and benthic foraminiferal calcite δ18O over the last eight glacial cycles. FB-δ15N is plotted decreasing upward, such that N2 fixation increases upward. FB-δ15N of O. universa (A−C, red) at MD97-2142 is similar to the FB-δ15N of G. ruber (A, blue) during the last 130 ky. Error bars indicate 1 SD of the oxidation replicates (Methods). FB-δ15N is highly correlated with the δ18O of G. ruber (B, gray) (64) and of C. wuellerstorfi (C, black). The glacial/interglacial change in the δ18O of the benthic foraminifera C. wuellerstorfi is typically considered to be mostly due to change in global ice volume (and hence sea level), with deep-water temperature change having a smaller effect. The light gray bars indicate the glacial terminations.
The FB-δ15N records have no clear correspondence with the bulk sediment records from the SCS, which do not show systematic glacial/interglacial changes (Fig. 2). Several of the existing bulk sediment records from the SCS are substantially dissimilar from one another (Fig. 2A) (27). Moreover, although foraminifera-bound N content is low and stable over glacial cycles, bulk sediment N content varies substantially over time and across records (Fig. 2B). Similar observations regarding SCS bulk sedimentary N records have previously been attributed to diagenesis and to multiple sources of N to the bulk sediment (28). Variation in terrigenous input at our study site has been documented to be associated with sea level change over the glacial cycles, for example, with higher concentrations of n-alkanes coinciding with lower sea level (29). A general disconnect between FB-δ15N and bulk sediment δ15N has been observed in the Caribbean Sea as well, where sedimentological data also point to terrestrial/shelf N inputs to the bulk sediments, especially in glacial intervals (13, 14). These findings argue against the utility of bulk sediment δ15N records for reconstructing the δ15N of export production in marine environments such as the SCS and Caribbean Sea, where terrestrial and shelf inputs are significant, export production is modest, and sedimentary organic matter preservation is not exceptionally high.
The FB-δ15N record from MD97-2142 indicates an increase in the δ15N of subsurface nitrate of the SCS during the glacials (Fig. 3). Throughout the ocean, the δ15N of subsurface nitrate is affected by lateral communication with other regions. Accordingly, one might propose that Pacific-wide processes raised the δ15N of the nitrate in the SCS by ∼3‰ during glacials. This might be driven by a whole ocean nitrate δ15N rise. Alternatively, it might be driven by a change in the rate of circulation in and out of the SCS and/or a change in N-cycle processes outside the SCS.
With regard to changes in lateral circulation, as described above, there is no clear mechanism by which communication with open western North Pacific waters shallower than ∼200 m could have a strong influence on SCS nitrate δ15N. Accordingly, this scenario must involve waters deeper than ∼200 m. However, modern oceanic nitrate isotope data do not indicate that a change in lateral circulation by itself would significantly change intermediate-depth nitrate δ15N in the SCS. For the western expanse of subtropical, subpolar, and tropical Pacific, even when including the western equatorial Pacific and existing measurements from the central South Pacific, the δ15N of nitrate in intermediate-depth waters falls between 5.5‰ and 7.0‰, with most measurements in a still narrower range (23, 24, 30–35). Intermediate-depth nitrate in the modern SCS falls squarely in this range (Fig. 1 and Fig. S1), in part because of the rapid lateral exchange of the SCS with the neighboring open western North Pacific through the Luzon Strait. If this weak variation in intermediate-depth nitrate δ15N also applied in the past, even major changes in the circulation of intermediate or mode waters would have had only modest effects on the δ15N of the nitrate imported into the SCS.
We next consider the possibility of global and/or Pacific-wide changes in nitrate δ15N that are communicated into the SCS. To compare with our record, we generated a 120-ky FB-δ15N record using Globigerinoides sacculifer from western tropical North Pacific ([Ocean Drilling Program (ODP) 807]. This new record as well as paired LGM and Holocene FB-δ15N data from the central equatorial Pacific (36) show only small δ15N differences between the LGM and the Holocene (Fig. 2). Bulk sediment records from the eastern Pacific show the opposite sense of δ15N change compared with that in the SCS (Fig. 4) (5, 37, 38). These and other records from across the global ocean argue against the possibility that the elevated FB-δ15N observed in the SCS during the LGM reflects a change in the δ15N of subsurface nitrate imported laterally from the open Pacific.
Fig. 4.
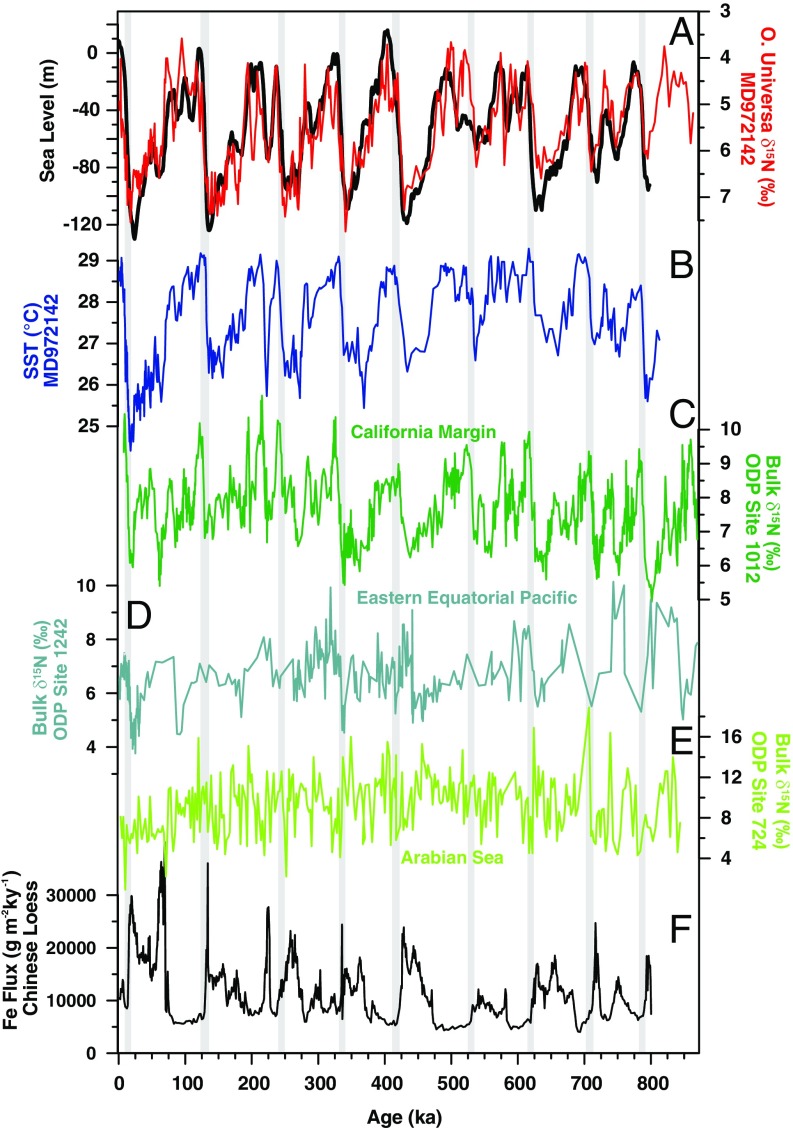
Records of N2 fixation, sea level, sea surface temperature, water column denitrification, and atmospheric iron supply. (A) Within the SCS, N2 fixation is generally higher (FB-δ15N is lower) in the interglacials (red; FB-δ15N increases downward). N2 fixation increases upon each termination and is tightly correlated with the mean relative sea level stack (black) generated using seven independent sea level reconstructions (49). (B) The structure of N2 fixation variability is also similar to changes in sea surface temperature at MD97-2142 (29) but lags behind temperature changes, especially during the latter half of the record. Among the three records sensitive to water column denitrification from (C) the California margin (37), (D) eastern Equatorial Pacific (65), and (E) Arabian Sea (66), the bulk δ15N record from the California margin is (negatively) correlated with FB-δ15N in the SCS (note reversal of δ15N scales between A and C−E). (F) A Chinese loess record shows generally higher iron flux in the glacials (67), which would increase N2 fixation during glacials if iron availability were the primary control for N2 fixation, inconsistent with the FB-δ15N data. The light gray bars indicate the glacial terminations.
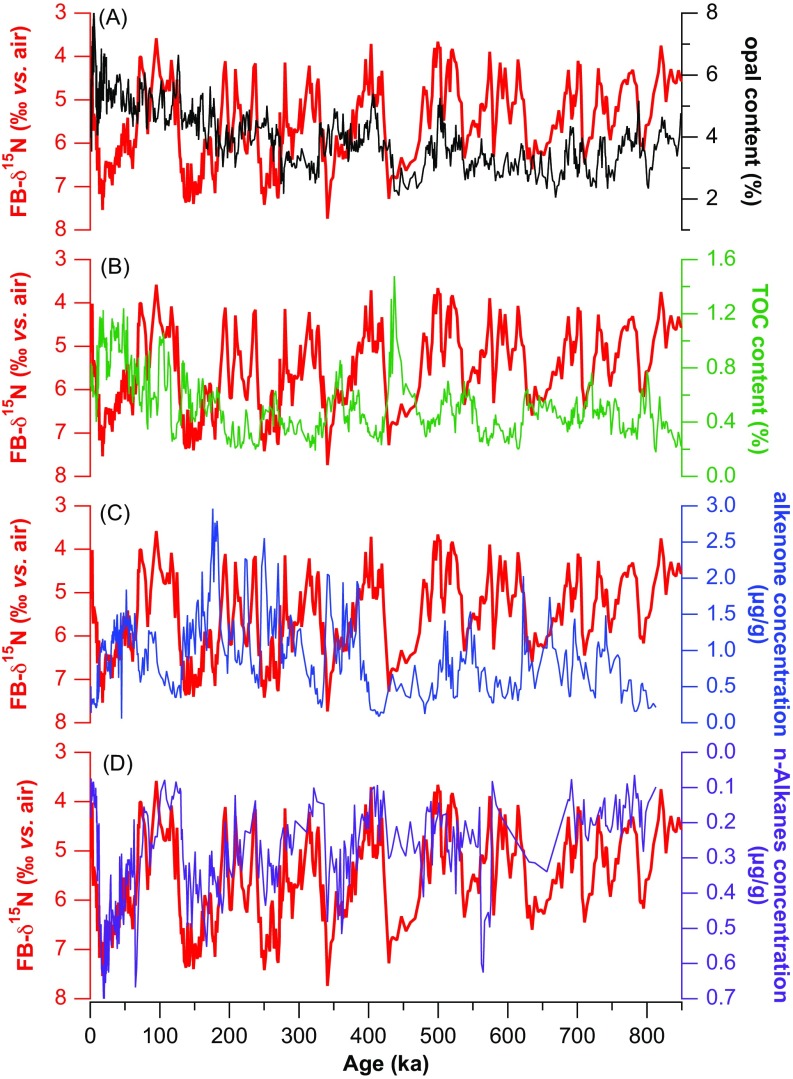
One might hypothesize greater vertical mixing in the SCS during ice ages, which might weaken the δ15N decline upward through the SCS thermocline, thus increasing the δ15N of the nitrate supply to the euphotic zone. However, this mechanism would predict simultaneous changes in productivity and FB-δ15N in the oligotrophic SCS, and yet the productivity proxies are not particularly well correlated with FB-δ15N (Fig. S3). Moreover, because deep thermocline waters have a substantially lower N/P ratio than the shallow thermocline waters (16), an increase in the supply of deeper-held nutrients to the surface would have encouraged an increase in N2 fixation, which would have worked to lower the δ15N of the sinking flux and of the shallow subsurface nitrate. This increase in N2 fixation would have countered the tendency for increased vertical mixing to raise the δ15N of the nitrate supply and, in turn, FB-δ15N. Finally, if changes in vertical mixing were the dominant driver of the δ15N changes, we would expect synchronous changes in the sea surface temperature (SST) and δ15N, which is not supported by our data (Figs. 3, 4, and 5A). Similarly, it is observed that a planktonic foraminiferal index of vertical mixing (39) changes early in the deglaciation and then stabilizes, whereas FB-δ15N evolves through the deglaciation and Holocene (Fig. 2) (40). As the effect of vertical exchange on nitrate δ15N would be essentially instantaneous (decadal at most), this lag argues against SCS hydrographic conditions as the dominant signal in FB-δ15N.
Fig. S3.
In MD972142, the FB-δ15N record in comparison with multiple productivity proxies [(A) opal content, (B) total organic carbon (TOC) content, and (C) alkenone concentration] and (D) with the concentration of n-alkanes (29), a biomarker of terrestrial input.
Fig. 5.
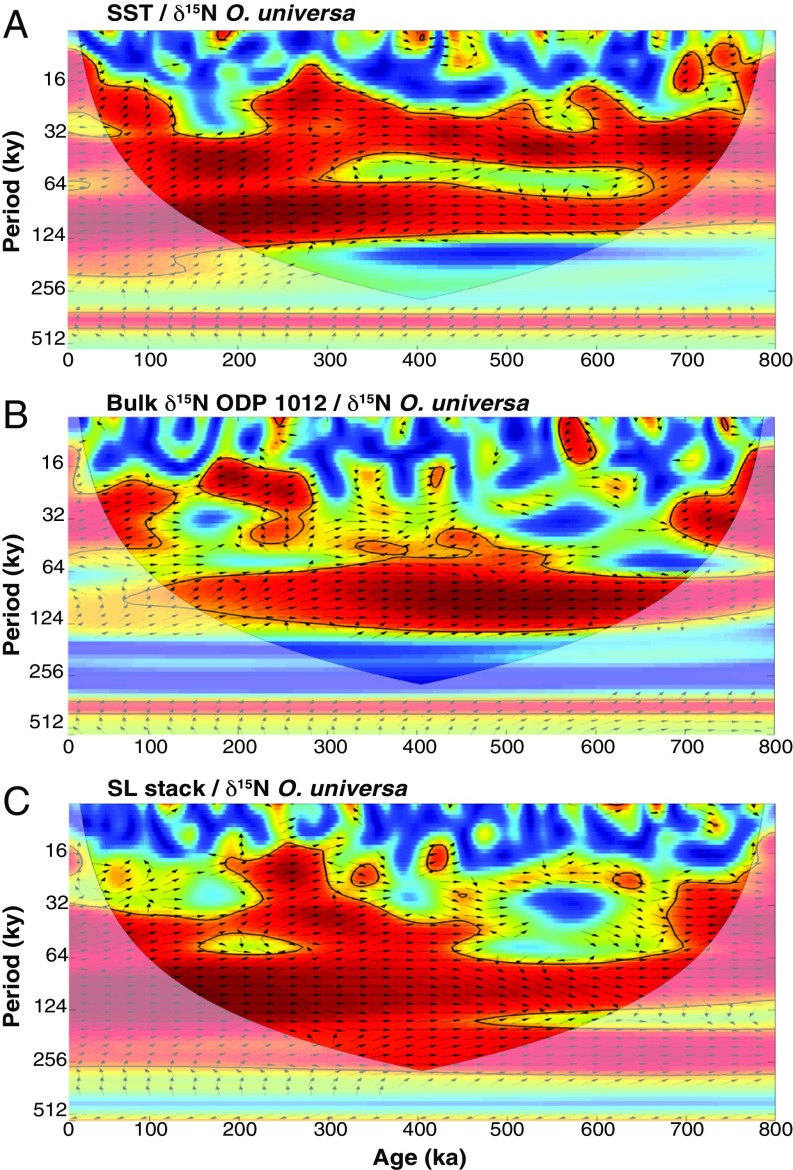
Cross-wavelet coherence and phase relationship among records of N2 fixation, sea level, sea surface temperature, and water column denitrification. Squared wavelet coherence between two time series was computed using the methods of ref. 68. The 95% confidence level against red noise was calculated using the Monte Carlo method and is shown as a thick contour that encloses the significant sections. The light shading indicates the region possibly influenced by edge effects. Black arrows indicate the phase relationship between the two time series, with in-phase pointing right, FB-δ15N leading a given climate variable pointing down, and FB-δ15N lagging pointing up. The different records have been interpolated to an evenly spaced time series of 2 ky before the spectral analysis. (A) The SST record (29) from the same sediment core has high coherency with, but leads, the FB-δ15N of O. universa by around 4 ky during the last 400 ky at the dominant 41- and 100-ky bands, as indicated by the direction of the arrows, which is inconsistent with a causal connection in this case. (B) The bulk δ15N record from California margin (37) is coherent with FB-δ15N in the SCS at the period near 100 ky. (C) The sea level record stack (47–49) shows high coherency with FB-δ15N at a wide range of frequencies.
Nitrogen inputs from river and atmospheric sources are also unlikely to explain the FB-δ15N variations. Clear signs of riverine N input are confined to the inner shelf above 30 m, and our preliminary data from two summer cruises show high δ15N values for the shallow shelf nitrate (up to 12‰). Atmospheric N deposition is low in δ15N relative to oceanic nitrate (41), so an increase in deposition would have been required during interglacials to explain the low FB-δ15N. However, the interglacial δ15N impact, when neglecting the recent rise in anthropogenic N, is far too low for its removal to have caused a 3‰ rise in FB-δ15N during ice ages (42, 43).
A changing rate of N2 fixation is the sole remaining mechanism with the potential to explain the cycles in FB-δ15N at this site in the SCS. We conclude that the δ15N of the shallow thermocline nitrate was lowered less by N2 fixation during glacials, due to an ice age reduction in the rate of this process. The amplitude of the SCS δ15N rise in the glacials is similar to that observed in the tropical western North Atlantic (13, 14), where N2 fixation also has a strong imprint on thermocline nitrate δ15N (20). The 3‰ amplitude of the glacial/interglacial FB-δ15N change in the SCS is comparable to the largest regional declines in ocean nitrate δ15N attributed to N2 fixation in the modern ocean (44, 45); this suggests that the ice age decline in N2 fixation rate was dramatic, most likely to less than half of the modern rate based on a two end-member mixing calculation (Estimate for Glacial–Interglacial Changes in N2 Fixation Rate).
A question that arises is how FB-δ15N glacial−interglacial variations of ∼3‰ could result when the modern nitrate δ15N decline from ∼500 m depth into the shallow SCS thermocline is only 1 to 2‰ (Fig. 1B). First, the Holocene does not represent the minimum observed FB-δ15N, so shallow thermocline nitrate δ15N is reconstructed to have been still lower during previous interglacials. Second, the role of N2 fixation in lowering the δ15N of subsurface nitrate is greater than indicated by the local vertical gradient in nitrate δ15N alone, as low δ15N N from N2 fixation spreads horizontally and vertically, as nitrate and sinking particulate nitrogen (45). This latter point also reinforces the arguments above against a hydrographic (e.g., vertical mixing) explanation for the observed FB-δ15N changes.
At all nine glacial terminations covered by our FB-δ15N record, a reconstructed increase in N2 fixation in the SCS coincides with decreases in planktonic and benthic δ18Oc, a rise in sea level and thus an increase in shelf area (Fig. 4 and Figs. S4 and S5), a rise in SST, and an apparent deglacial increase in water column denitrification in the eastern tropical Pacific (Figs. 3 and 4). The length of the SCS FB-δ15N record allows for the use of time series analysis to identify the correlations that are most consistent with a causal connection.
Fig. S4.
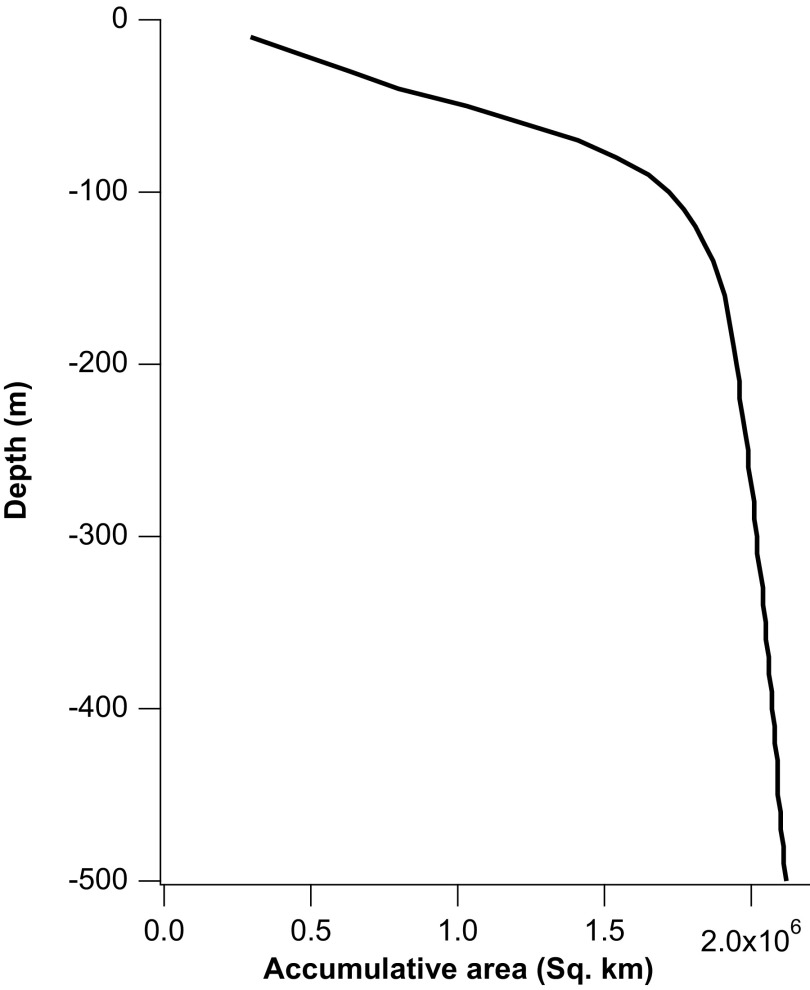
The SCS hypsographic curve generated using the ETOPO1 Ice Surface global relief model (73).
Fig. S5.
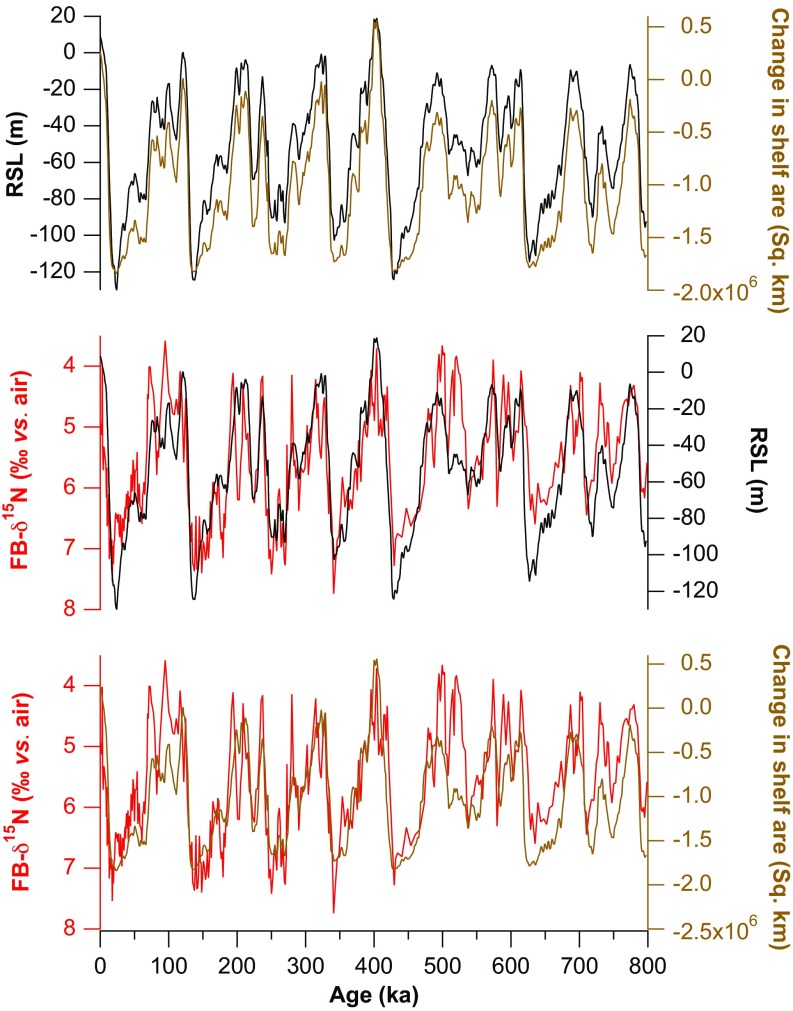
Comparison of the FB-δ15N record with calculated ice volume-driven changes. Because of the nearly linear relationship between sea level and shelf area in the SCS within the upper 100 m, changes in relative sea level (RSL) and shelf area are almost synchronous over the past glacial/interglacial cycles.
Variability in SST is highly coherent with that in FB-δ15N (Fig. 5A). However, FB-δ15N lags SST by more than 4 ky in the dominant 41- and 100-ky bands for the latter half of the record (Fig. 5A). Because the physiological and biochemical response of N2 fixers to SST would be effectively instantaneous, the lag argues against SST as the driver of the greatest FB-δ15N variations. Moreover, based on observed sensitivities (15), the reconstructed SCS SSTs fall into the optimal range for N2 fixation, and a 3 °C cooling would be far too small to explain the dramatic reduction in N2 fixation during glacials. Dust fluxes are lowest when reconstructed N2 fixation is highest, arguing against iron supply as the explanation for the reconstructed N2 fixation changes (Fig. 4F). This lack of positive correlation between N2 fixation and dust supply is consistent with high iron availability in the SCS even during interglacials, both from the margins and from atmospheric deposition.
There are three bulk sediment δ15N records from near water column zones of suboxia and that are adequately long to compare with our SCS FB-δ15N record (Figs. 4 C−E and 5B). These environments are characterized by high export production and relatively good preservation of sedimentary organic matter, such that the potential of bulk sediment δ15N to robustly record the δ15N of N export is greater than in most other ocean regions (46). Of these records, only ODP Site 1012 (37) from the California margin shows significant coherency (Figs. 4C and 5B). The anticorrelation of the records might be taken to suggest that enhanced water column denitrification in the eastern tropical North Pacific during interglacials was responsible for coincident N2 fixation in the SCS. However, the coherency is limited to periods near 100 ky, suggesting that observed similarities in the records reflect independent but similarly timed responses to glacial cycles.
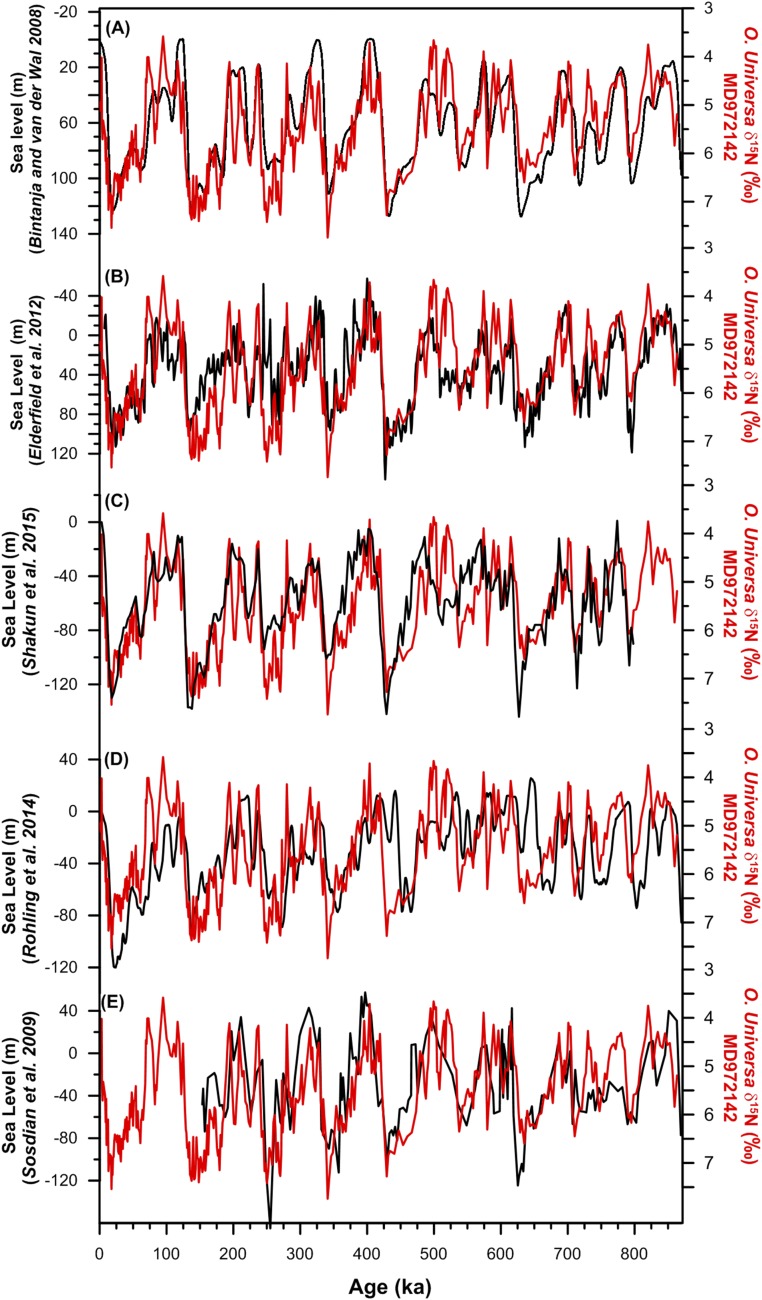
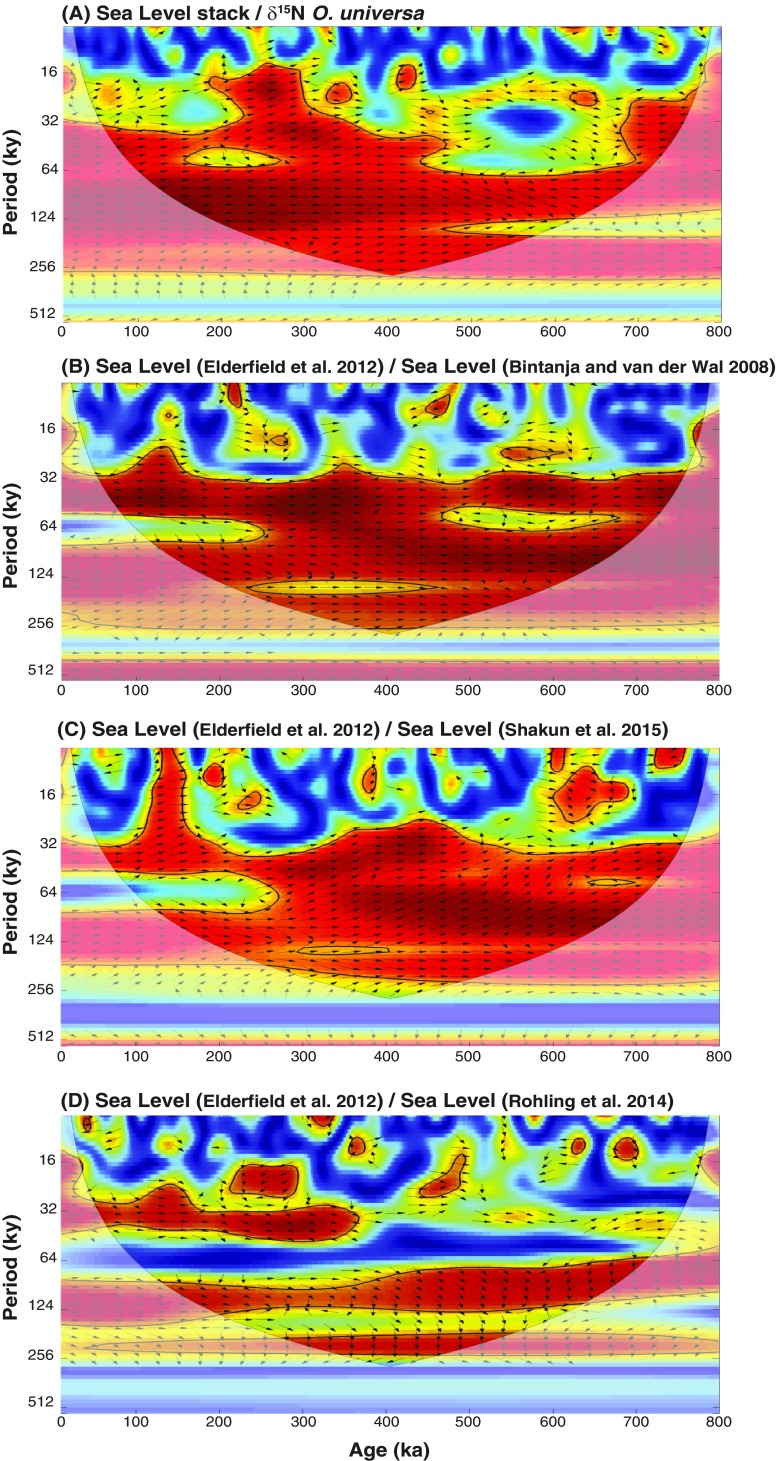
The SCS FB-δ15N and δ18Oc records are similar in large-scale structure (Figs. 3 B and C), suggesting a connection between N2 fixation and sea level. A stack of sea level records (47–49) shows high coherency with the SCS FB-δ15N over a wide range of frequencies (Fig. 5C; significant against red noise with 95% confidence), as strong as the coherency between independent sea level reconstructions (Figs. S6−S8). Thus, the reconstructed glacial/interglacial changes in N2 fixation appear to require a mechanism that involves ice volume and/or sea level change. The correlation of markers of terrigenous input with FB-δ15N in MD972142, with greater terrigenous material when FB-δ15N is high (29), provides additional support for this interpretation (Fig. S3D). As no relatively direct, low-lag connection between ice volume and N2 fixation appears plausible for the SCS, the data argue for sea level as the dominant driver of N2 fixation change.
Fig. S6.
The FB-δ15N record in comparison with different sea level records. FB-δ15N is plotted with the five individual sea level records (A–E) that are part of the sea level stack and that cover the last 800 ky (47, 48, 75–77).
Fig. S8.
Cross-wavelet coherence and phase relationship of the FB-δ15N and benthic δ18O at MD972142. Squared wavelet coherence between two time series was computed using the methods proposed by ref. 68. This analysis identifies regions of significant (95% confidence) coherence and its relative phase in time−frequency space. The 95% confidence level against red noise was calculated using the Monte Carlo method and is shown as a thick contour that encloses the significant sections. The light shading represents the COI, which ensures that the edge effects are negligible beyond this point. Black arrows indicate the relative phase relationship between the two time series, with in-phase pointing right, antiphase pointing left, benthic δ18O leading FB-δ15N by 90° pointing up, and benthic δ18O lagging by 90° pointing down. The different records have been interpolated to an evenly spaced time series of 2 ky before the spectral analysis of the data. A lag of FB-δ15N relative to benthic foraminiferal δ18O is observed especially in the four most recent terminations, as indicated by the direction of the arrows over the past 400 ky at the dominant 100-ky period.
Fig. S7.
Cross-wavelet coherence and phase relationship of the sea level record from ODP site 1123 (47) and other sea level records (48, 75, 76) that cover the past 800 ky. Squared wavelet coherence between two time series was computed using the methods proposed by ref. 68. This analysis identifies regions of significant (95% confidence) coherence and its relative phase in time−frequency space. The 95% confidence level against red noise was calculated using the Monte Carlo method and is shown as a thick contour that encloses the significant sections. The light shading represents the cone of influence (COI), which ensures that the edge effects are negligible beyond this point. Black arrows indicate the relative phase relationship between the two time series, with in-phase pointing right, antiphase pointing left, ODP 1123 Sea Level (47) leading a given sea level record by 90° pointing up, and ODP 1123 Sea Level lagging by 90° pointing down. The different records have been interpolated to an evenly spaced time series of 2 ky before the spectral analysis of the data. The results indicate that the coherency of the SCS FB-δ15N record with (A) the sea level stack is as strong as the coherency among (B−D) the independent sea level reconstructions.
The extensive continental shelf area of the tropical western North Pacific adjacent to the SCS, the Sunda shelf in particular, appears to be an important locus of benthic denitrification (8). This shelf area was nearly completely lost during peak glacials (Fig. 1A). The reduction in shelf area has been proposed to reduce shelf sedimentary denitrification in the glacials (7), which, in turn, would lead to higher N/P (less excess P) in the upper water column. This change would have discouraged N2 fixation in the SCS and neighboring regions, explaining the remarkable coherency of the sea level records and our SCS FB-δ15N record (Fig. 6).
Fig. 6.
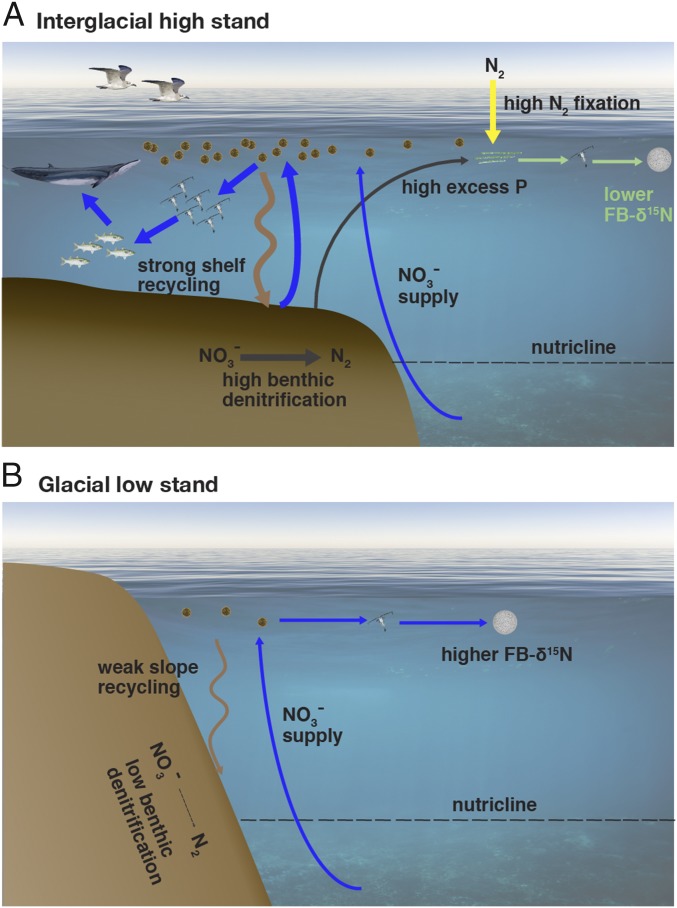
Inferred glacial/interglacial changes along the SCS margin. (A) During interglacial high sea level stands, organic matter decomposition on the shallow shelf promotes high coastal ocean productivity and rapid shelf denitrification. The denitrification, by consuming fixed N, causes the shelf water to have excess P. When this water is transported into the open SCS, phytoplankton growth draws down its nutrients, and its excess P causes N to become depleted before P. The availability of P in the absence of N enhances N2 fixation, which is reflected in a lowering of thermocline nitrate δ15N and thus lower FB-δ15N. (B) The sea level-driven loss of the shallow shelf during glacials reduces productivity and sedimentary denitrification along the margin. The reduction in sedimentary denitrification rate is compensated by slower offshore N2 fixation, causing thermocline nitrate δ15N and FB-δ15N to rise. Along the margin, the glacial reduction in shallow seafloor nutrient recycling and thus phytoplankton production would impact the upper trophic levels that thrive on the modern (interglacial) shelf. This mechanism, which explains the observed coupled changes in sea level and N2 fixation in the SCS, should also apply along other ocean margins.
The SCS FB-δ15N record thus provides the most direct evidence to date for the long-hypothesized scenario in which sea level drives glacial cycles in benthic N loss along the continental margins. Such a mechanism implies that SCS N2 fixation responded to changes in nearby shelf area, as changes in N loss on distant shelves should have been compensated by N2 fixation in those regions. N2 fixation compensation for N loss might be confounded by changes in iron availability in other tropical/subtropical ocean regions. However, for regions such as the SCS that are characterized by high iron supply, local compensation for N loss changes is arguably to be expected.
Continental slopes are known to deposit substantial quantities of margin-derived organic matter at their base (50), and the resulting accumulation drives denitrification on the slope (31, 51, 52). It is possible that this process was accelerated during ice ages and, in part, replaced the sedimentary denitrification on the continental shelves. N loss on the slope may not lead to synchronous changes in N2 fixation because the N deficit would accumulate in deep water, not directly affecting the N/P of the nutrient supply to the locally overlying surface ocean. However, the funneling of organic matter into the deep ocean prevents the upper ocean nutrient recycling and other processes that render N loss so rapid on the shelves. Therefore, any increased N loss by denitrification on the slope is unlikely to have substantially compensated for the reduced N loss on the shallow margins.
N2 fixation slowed substantially during ice ages, as reconstructed here for the western tropical Pacific and previously for the North Atlantic, in both cases consistent with the response of N2 fixation to excess P supply as the dominant driver of the changes (13, 14). The correlation between SCS N2 fixation and sea level provides data-based support for the hypothesis of reduced sedimentary denitrification during ice ages (7, 53, 54), and bulk sediment δ15N records argue for reductions in water column denitrification as well (5, 6). With these lower rates of both input and loss, the residence time of fixed N in the ocean [currently ∼3 ky (55, 56)] would have become longer and thus less distinct from the residence time of phosphorus [15 ky to 40 ky (57)], although the latter may also have changed over glacial cycles.
Benthic N loss on the continental margins reflects the high flux of organic matter to the coastal seabed (50–52), a consequence of both the shallow continental shelf and the high productivity of the coastal water column (Fig. 6). The high productivity is, in turn, supported by the shelf, which traps sinking organic matter and quickly returns nutrients to the sunlit surface ocean. Thus, the reduction in benthic N loss during ice ages implies a net decline in the organic matter supply to coastal ecosystems, especially those organisms that rely on the benthos. In part because of their extraordinarily high productivity and benthic activity, the modern continental shelves have tremendous importance for seafloor fauna, fish, and marine mammals. The reconstructed biogeochemical changes imply that these higher trophic levels would have suffered a notable decline in food supply during the low sea level stands of ice ages (Fig. 6), potentially impacting the evolution and current characteristics of coastal species and ecosystems (e.g., ref. 58).
Estimate for Glacial–Interglacial Changes in N2 Fixation Rate
The deep thermocline nitrate in the SCS is transported from the western Pacific Ocean primarily through Luzon Strait. In the tropical latitudes of the western and central Pacific, nitrate in the lower thermocline (300 m to 600 m depth) has a δ15N between 7‰ and 8‰, with most of the nitrate between 7.0‰ and 7.5‰ (24, 32). Newly fixed N from N2 fixation, on the other hand, has a low δ15N close to that of atmospheric N2 δ15N (−2‰ to 0‰ vs. air N2) (69–71), and it is taken as the dominant mechanism that lowers nitrate δ15N in the subsurface of the SCS and nearby regions to values below 7‰ (22). The current shallow subsurface nitrate δ15N at the northern SCS is 4.9‰ [average nitrate δ15N at 100 m depth from four cruises from 2012 to 2013 at SEATS) (Fig. 1C)]. Using a two end-member mixture of newly fixed N with a δ15N of −1‰ and the deep thermocline nitrate with a δ15N of 7‰ in the SCS [δ15NN2 fixation × FractionN2 fixation + δ15Nthermocline nitrate × (1 − FractionN2 fixation) = δ15Nsubsurface nitrate], we estimate that about 26% of the nitrate at 100 m depth originates from N2 fixation in the SCS and the neighboring western Pacific margin.
The δ15N of both G. ruber and O. universa measured in the surface sediment is 4.9‰, the same as the 100-m-depth minimum in nitrate δ15N (Fig. 1C). This nitrate-to-foraminifera similarity is consistent with our previous findings from global compilations that FB-δ15N is similar to the δ15N of the subsurface nitrate available for upward transport into the euphotic zone. We thus use past changes in the FB-δ15N to reconstruct past changes in the subsurface nitrate δ15N, and, in turn, changes in N2 fixation rate. Alternative explanations for the FB-δ15N changes involving circulation- or remote biogeochemistry-driven changes in the δ15N of the interior nitrate supplied to the SCS are discussed in Results and Discussion.
FB-δ15N varies between 4‰ and 7‰ from peak interglacials to glacials. Using the average FB-δ15N of G. ruber between 21 ka and 29 ka (∼6.0‰), we estimate that recently fixed N contributes only about 13% of the shallow subsurface nitrate during the LGM. If we assume that the higher FB-δ15N of the LGM is due to changes in N2 fixation alone, then the LGM N2 fixation rate is only 48% of its Holocene rate. In our previous study, this value is estimated to be 27% (40); this is because we had assumed that the intermediate-depth nitrate δ15N (∼6.2‰) in the SCS was not affected by N2 fixation. However, the intermediate-depth nitrate δ15N in the SCS is lower than in the open western and central Pacific, suggesting that the entire water column in the SCS is influenced by nitrate from the remineralization of newly fixed N; this is to be expected given the strong vertical mixing in the deep SCS (72). The minimum interglacial FB-δ15N value of 4‰ suggests that newly fixed N can contribute up to 38% of the subsurface nitrate. In summary, our record indicates that the N2 fixation rate in the SCS varies by threefold between ice ages and interglacials.
Methods
FB-δ15N Analyses.
The protocol follows and is modified from that of refs. 13 and 14. The individual foraminifera species (250- to 425-μm-size fraction, ∼5 mg per sample) are picked manually and gently crushed under a dissecting microscope. Samples are first sonicated for 5 min in an ultrasonic bath using 2% polyphosphate solution to remove clay particles. To remove metal coatings, bicarbonate-buffered dithionite−citric acid solution is then added to each sample, and the samples are placed in a water bath at 80 °C for 1 h. The final cleaning step is oxidative: Basic potassium persulfate solution is added to each sample, and the samples are autoclaved (at 121 °C) for 1 h. The cleaned samples are rinsed in deionized water and dried overnight at 55 °C. This cleaning protocol typically preserves 60 to 75% of the initial foraminifera weight.
Cleaned foraminifera (∼3 mg to 4 mg per sample) are weighed into a previously combusted glass vial and dissolved in 3N HCl. To convert the released organic N to nitrate, purified basic potassium persulfate oxidizing solution is added to the vials, which are then autoclaved for 1 h on a slow-vent setting. To lower the N blank associated with the oxidizing solution, the potassium persulfate is recrystallized three times. At the time of processing, 0.8 g of NaOH and 0.5 g of potassium persulfate are dissolved in 100 mL of deionized water. Organic standards are used to constrain the δ15N of the persulfate reagent blank. Three different organic standards were used: US Geological Survey (USGS) 40 (δ15N = −4.5‰ vs. air), USGS 41 (δ15N = 47.6‰ vs. air), and a laboratory standard made of a mixture of 6-aminocaproic acid and glycine (δ15N = 5.4‰ vs. air). A minimum of 18 organic standards and three to five blanks were analyzed per batch of samples.
To determine the N content of the samples, nitrate concentration is measured in the oxidation solution after autoclaving. The nitrate analysis is by reduction to nitric oxide using vanadium (III) followed by chemiluminescence detection (59). The blank is also quantified in this way. Consistent with our previous findings, O. universa and G. ruber had an average N content of 3 mmol to 4 mmol N per gram of cleaned calcite, yielding nitrate concentrations in the oxidation solutions of 10 µM to 20 µM, whereas the nitrate concentration of the blanks ranged between 0.3 µM and 0.7 µM (less than 5%, typically less than 2%, of the total N per sample).
The δ15N of the samples is determined using the denitrifier method in conjunction with gas chromatography and isotope ratio mass spectrometry (60, 61). The denitrifier method involves the transformation of dissolved nitrate and nitrite into nitrous oxide gas (N2O) via a naturally occurring denitrifying bacterial strain that lacks an active form of the enzyme N2O reductase. Before adding the foraminifera samples to the bacteria, the sample solution is acidified to pH 3 to 7. The denitrifier Pseudomonas chlororaphis was used for this work. Normally, 5-nmol samples are added to 1.5 mL of bacterial concentrate after degassing of the bacteria. Along with the samples, the organic standards as well as replicate analyses of nitrate reference material International Atomic Energy Agency NO3 reference (IAEA-N3) (δ15N = 4.7‰ vs. air) and a bacterial blank are also measured. The IAEA-N3 standards are used to monitor the bacterial conversion and the stability of the mass spectrometry, and the oxidation standards are used to correct for the oxidation blanks. If possible, samples were oxidized in duplicate, and oxidized samples were also sometimes analyzed by the denitrifier method in duplicate. The denitrifier method typically has a SD (1σ) of less than 0.1‰ and is not reported here. The reported error is the SD estimated from the means of separate oxidations of cleaned foraminiferal material, which averaged 0.22‰ (57% were less than 0.2‰, and 93% were less than 0.5‰).
The data reported in this work will be accessible at National Centers for Environmental Information (NOAA) once the paper is published online.
The δ18O Analyses on Cibicidoides wuellerstorfi.
Approximately 15 Cibicidoides wuellerstorfi individuals were picked from each sample. The samples were ultrasonicated first in 1 mL of deionized water for 3 s to 5 s, then in 0.2 mL of methanol for 3 s to 5 s. The samples were rinsed with deionized water two to three times and dried in an oven at 60 °C overnight. The cleaned foraminifera samples were crushed, and 35 mg to 80 mg weighed into 4.5-mL vials. The δ18O were analyzed with a Thermo GasBench II coupled to a Thermo Delta V Plus mass spectrometer at Eidgenössische Technische Hochschule Zürich (62). The average of the SD of single δ18O measurements is ∼0.04%.
Nitrate Sampling and δ15N Analyses at the South East Asian Time-Series Station and in the Open Western Pacific.
The South East Asian Time-Series (SEATS) station is located at 18°N and 116°E (Fig. 1A) in about 3,800 m of water. It was sampled four times between August 2012 and December 2013 in approximately seasonal intervals aboard R/V Ocean Researcher I. Two casts during August 2012 and eight casts from each of the other three cruises were sampled for nitrate δ15N analyses. The western subtropical Pacific transect is located along 23.5°N from 122.25°E to 126°E. Discrete water samples were collected from five open ocean stations in 2013 July on R/V Ocean Research V. All water samples were collected with General Oceanics GO-FLO bottles bottles mounted onto a Rosette sampling assembly. From each depth, seawater was collected unfiltered in a rinsed 60-mL high-density polyethylene bottle and immediately frozen at −20 °C.
The concentration of nitrate plus nitrite was analyzed by reduction to nitric oxide using vanadium (III) followed by chemiluminescence detection (59). The δ15N of nitrate was determined using the denitrifier method, as described above. We use two international nitrate isotope reference materials, IAEA-N3 (δ15N = 4.7‰ vs. air) and USGS-34 (δ15N = −1.8‰ vs. air), to correct the data. The analytical precision for δ15N was 0.08‰. The error bars in Fig. 1C represent 1 SD of the nitrate δ15N analyzed at the same depth from the different casts, which averaged 0.20‰.
Phase Relationship Between FB-δ15N and Benthic Calcite δ18O
FB-δ15N and benthic calcite δ18O show high coherency throughout the record. One possible exception involves the major glacial terminations (such as that before the Holocene; Fig. 3C and Fig. S8), during which a transient nitrate δ15N rise of ∼1‰ may occur (40, 54). This δ15N rise would introduce an apparent lag in the deglacial FB-δ15N decrease relative to whatever environmental parameter is predominantly driving the glacial/interglacial changes, potentially explaining the observed lag of FB-δ15N relative to sea level at the terminations.
Shelf Area and Sea Level in the SCS
The SCS hypsographic curve [using the ETOPO1 Ice Surface global relief model (73)] suggests that the shallow shelf area and sea level is close to linearly correlated within the upper 100 m (Fig. S4). As a result, changes in sea level and shelf area would have been almost synchronous, with little deviation from a linearly proportional relationship (Fig. S5).
Acknowledgments
We thank M. A. Weigand and S. Oleynik for assistance with the nitrate and foraminifera-bound δ15N analyses; Tung-Yuan Ho, Kuo-Yuan Lee, and Yao-Chu Wu for their assistance on cruises and water sampling; and M. P. Hain for discussions. Funding was provided by Taiwan Ministry of Science and Technology (MOST) Grant 105-2628-M-002-007-MY3, by National Taiwan University Grant NTU-CESRP-106R7625-1 (to H.R.), by MOST Grant NSC 101-2611-M-001-003-MY3 and a grant from the Sustainability Science Research Program of the Academia Sinica (to G.T.F.W.), by the US National Science Foundation through Grants OCE-1060947 and PLR-1401489 (to D.M.S.), and by the Grand Challenges Program of Princeton University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this work have been deposited with National Centers for Environmental Information (NOAA), https://www.ncdc.noaa.gov (accession no. 22390).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701315114/-/DCSupplemental.
References
- 1.Moore JK, Lindsay K, Doney SC, Long MC, Misumi K. Marine ecosystem dynamics and biogeochemical cycling in the Community Earth System Model [CESM1(BGC)]: Comparison of the 1990s with the 2090s under the RCP4.5 and RCP8.5 Scenarios. J Clim. 2013;26:9291–9312. [Google Scholar]
- 2.Gruber N, Galloway JN. An Earth-system perspective of the global nitrogen cycle. Nature. 2008;451:293–296. doi: 10.1038/nature06592. [DOI] [PubMed] [Google Scholar]
- 3.Falkowski PG. Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature. 1997;387:272–275. [Google Scholar]
- 4.Broecker WS, Henderson GM. The sequence of events surrounding Termination II and their implications for the cause of glacial-interglacial CO2 changes. Paleoceanography. 1998;13:352–364. [Google Scholar]
- 5.Ganeshram RS, Pedersen TF, Calvert SE, Murray JW. Large changes in oceanic nutrient inventories from glacial to interglacial periods. Nature. 1995;376:755–758. [Google Scholar]
- 6.Altabet MA, Francois R, Murray DW, Prell WL. Climate-related variations in denitrification in the Arabian Sea from sediment 15N/14N ratios. Nature. 1995;373:506–509. [Google Scholar]
- 7.Christensen JP. Carbon export from continental shelves, denitrification and atmospheric carbon dioxide. Cont Shelf Res. 1994;14:547–576. [Google Scholar]
- 8.Bianchi D, Dunne JP, Sarmiento JL, Galbraith ED. Data-based estimates of suboxia, denitrification, and N2O production in the ocean and their sensitivities to dissolved O2. Glob Biogeochem Cycles. 2012;26:GB2009. [Google Scholar]
- 9.Brandes JA, Devol AH. Isotopic fractionation of oxygen and nitrogen in coastal marine sediments. Geochim Cosmochim Acta. 1997;61:1793–1801. [Google Scholar]
- 10.Lehmann MF, Sigman DM, Berelson WM. Coupling the N-15/N-14 and O-18/O-16 of nitrate as a constraint on benthic nitrogen cycling. Mar Chem. 2004;88:1–20. [Google Scholar]
- 11.Sañudo-Wilhelmy SA, et al. Phosphorus limitation of nitrogen fixation by Trichodesmium in the central Atlantic Ocean. Nature. 2001;411:66–69. doi: 10.1038/35075041. [DOI] [PubMed] [Google Scholar]
- 12.Deutsch C, Sarmiento JL, Sigman DM, Gruber N, Dunne JP. Spatial coupling of nitrogen inputs and losses in the ocean. Nature. 2007;445:163–167. doi: 10.1038/nature05392. [DOI] [PubMed] [Google Scholar]
- 13.Ren H, et al. Foraminiferal isotope evidence of reduced nitrogen fixation in the ice age Atlantic Ocean. Science. 2009;323:244–248. doi: 10.1126/science.1165787. [DOI] [PubMed] [Google Scholar]
- 14.Straub M, et al. Changes in North Atlantic nitrogen fixation controlled by ocean circulation. Nature. 2013;501:200–203. doi: 10.1038/nature12397. [DOI] [PubMed] [Google Scholar]
- 15.Houlton BZ, Wang YP, Vitousek PM, Field CB. A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature. 2008;454:327–330. doi: 10.1038/nature07028. [DOI] [PubMed] [Google Scholar]
- 16.Wong GTF, Ku T-L, Mulholland M, Tseng C-M, Wang D-P. The SouthEast Asian Time-series Study (SEATS) and the biogeochemistry of the South China Sea—An overview. Deep Sea Res Part II Top Stud Oceanogr. 2007;54:1434–1447. [Google Scholar]
- 17.Zhang R, et al. Physical-biological coupling of N2 fixation in the northwestern South China Sea coastal upwelling during summer. Limnol Oceanogr. 2015;60:1411–1425. [Google Scholar]
- 18.Wada E, Terazaki M, Kabaya Y, Nemoto T. 15N and 13C abundances in the Antartic Ocean with emphasis on the biogeochemical structure of the food web. Deep Sea Res Part A Oceanogr Res Pap. 1987;34:829–841. [Google Scholar]
- 19.Brandes JA, Devol AH, Yoshinari T, Jayakumar DA, Naqvi SWA. Isotopic composition of nitrate in the central Arabian Sea and eastern tropical North Pacific: A tracer for mixing and nitrogen cycles. Limnol Oceanogr. 1998;43:1680–1689. [Google Scholar]
- 20.Knapp AN, DiFiore PJ, Deutsch C, Sigman DM, Lipschultz F. Nitrate isotopic composition between Bermuda and Puerto Rico: Implications for N2 fixation in the Atlantic Ocean. Glob Biogeochem Cycles. 2008;22:GB3014. [Google Scholar]
- 21.Liu KK, Su MJ, Hsueh CR, Gong GC. The nitrogen isotopic composition of nitrate in the Kurosio Water northeast of Taiwan: Evidence for nitrogen fixation as a source of isotopically light nitrate. Mar Chem. 1996;54:273–292. [Google Scholar]
- 22.Wong GTF, et al. Nitrate anomaly in the upper nutricline in the northern South China Sea - Evidence for nitrogen fixation. Geophys Res Lett. 2002;29:2097. [Google Scholar]
- 23.Ren H, et al. Glacial-to-interglacial changes in nitrate supply and consumption in the subarctic North Pacific from microfossil-bound N isotopes at two trophic levels. Paleoceanography. 2015;30:1217–1232. [Google Scholar]
- 24.Rafter PA, Sigman DM, Charles CD, Kaiser J, Haug GH. Subsurface tropical Pacific nitrogen isotopic composition of nitrate: Biogeochemical signals and their transport. Glob Biogeochem Cycles. 2012;26:GB1003. [Google Scholar]
- 25.Chavez EP, Toggweiler JR. Physical Estimates of Global New Production: The Upwelling Contribution. Wiley; New York: 1995. [Google Scholar]
- 26.Ren H, Sigman DM, Thunell RC, Prokopenko MG. Nitrogen isotopic composition of planktonic foraminifera from the modern ocean and recent sediments. Limnol Oceanogr. 2012;57:1011–1024. [Google Scholar]
- 27.Kienast M. Unchanged nitrogen isotopic composition of organic matter in the South China Sea during the last climatic cycle: Global implications. Paleoceanography. 2000;15:244–253. [Google Scholar]
- 28.Kienast M, et al. On the sedimentological origin of down-core variations of bulk sedimentary nitrogen isotope ratios. Paleoceanography. 2005;20:PA2009. [Google Scholar]
- 29.Shiau LJ, et al. Sea surface temperature, productivity, and terrestrial flux variations of the southeastern South China Sea over the past 800000 years (IMAGES MD972142) Terr Atmos Ocean Sci. 2008;19:363–376. [Google Scholar]
- 30.Casciotti KL, Trull TW, Glover DM, Davies D. Constraints on nitrogen cycling at the subtropical North Pacific Station ALOHA from isotopic measurements of nitrate and particulate nitrogen. Deep Sea Res Part II Top Stud Oceanogr. 2008;55:1661–1672. [Google Scholar]
- 31.Lehmann MF, et al. Origin of the deep Bering Sea nitrate deficit: Constraints from the nitrogen and oxygen isotopic composition of water column nitrate and benthic nitrate fluxes. Glob Biogeochem Cycles. 2005;19:GB4005. [Google Scholar]
- 32.Kienast M, et al. A mid-Holocene transition in the nitrogen dynamics of the western equatorial Pacific: Evidence of a deepening thermocline? Geophys Res Lett. 2008;35:L23610. [Google Scholar]
- 33.Rafter PA, Difiore PJ, Sigman DM. Coupled nitrate nitrogen and oxygen isotopes and organic matter remineralization in the Southern and Pacific Oceans. J Geophys Res Oceans. 2013;118:4781–4794. [Google Scholar]
- 34.Rafter PA, Sigman DM. Spatial distribution and temporal variation of nitrate nitrogen and oxygen isotopes in the upper equatorial Pacific Ocean. Limnol Oceanogr. 2015;61:14–31. [Google Scholar]
- 35.Sigman DM, DiFiore PJ, Hain MP, Deutsch C, Karl DM. Sinking organic matter spreads the nitrogen isotope signal of pelagic denitrification in the North Pacific. Geophys Res Lett. 2009;36:L08605. [Google Scholar]
- 36.Costa KM, et al. No iron fertilization in the equatorial Pacific Ocean during the last ice age. Nature. 2016;529:519–522. doi: 10.1038/nature16453. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z, Altabet MA, Herbert TD. Plio-Pleistocene denitrification in the eastern tropical North Pacific: Intensification at 2.1 Ma. Geochem Geophys Geosyst. 2008;9:Q11006. [Google Scholar]
- 38.Studer AS, Ellis KK, Oleynik S, Sigman DM, Haug GH. Size-specific opal-bound nitrogen isotope measurements in North Pacific sediments. Geochim Cosmochim Acta. 2013;120:179–194. [Google Scholar]
- 39.Yu P-S, Huang C-C, Chin Y, Mii H-S, Chen M-T. Late Quaternary East Asian Monsoon variability in the South China Sea: Evidence from planktonic foraminifera faunal and hydrographic gradient records. Palaeogeogr Palaeoclimatol Palaeoecol. 2006;236:74–90. [Google Scholar]
- 40.Ren H, Sigman DM, Chen M-T, Kao S-J. Elevated foraminifera-bound nitrogen isotopic composition during the last ice age in the South China Sea and its global and regional implications. Glob Biogeochem Cycles. 2012;26:GB1031. [Google Scholar]
- 41.Yang JYT, Hsu SC, Dai M, Hsiao SSY, Kao SJ. Isotopic composition of water-soluble nitrate in bulk atmospheric deposition at Dongsha Island: sources and implications of external N supply to the northern South China Sea. Biogeosci Discuss. 2013;10:9661–9695. [Google Scholar]
- 42.Yang S, Gruber N. The anthropogenic perturbation of the marine nitrogen cycle by atmospheric deposition: Nitrogen cycle feedbacks and the 15N Haber‐Bosch effect. Glob Biogeochem Cycles. 2016;30:1418–1440. [Google Scholar]
- 43.Ren H, et al. 21st-century rise in anthropogenic nitrogen deposition on a remote coral reef. Science. 2017;356:749–752. doi: 10.1126/science.aal3869. [DOI] [PubMed] [Google Scholar]
- 44.Sigman DM, et al. The dual isotopes of deep nitrate as a constraint on the cycle and budget of oceanic fixed nitrogen. Deep Sea Res Part I Oceanogr Res Pap. 2009;56:1419–1439. [Google Scholar]
- 45.Marconi D, et al. Nitrate isotope distributions on the US GEOTRACES North Atlantic cross-basin section: Signals of polar nitrate sources and low latitude nitrogen cycling. Mar Chem. 2015;177:143–156. [Google Scholar]
- 46.Altabet MA, et al. The nitrogen isotope biogeochemistry of sinking particles from the margin of the Eastern North Pacific. Deep Sea Res Part I Oceanogr Res Pap. 1999;46:655–679. [Google Scholar]
- 47.Elderfield H, et al. Evolution of ocean temperature and ice volume through the mid-Pleistocene climate transition. Science. 2012;337:704–709. doi: 10.1126/science.1221294. [DOI] [PubMed] [Google Scholar]
- 48.Bintanja R, van de Wal RS. North American ice-sheet dynamics and the onset of 100,000-year glacial cycles. Nature. 2008;454:869–872. doi: 10.1038/nature07158. [DOI] [PubMed] [Google Scholar]
- 49.Spratt RM, Lisiecki LE. A Late Pleistocene sea level stack. Clim Past. 2016;12:1079–1092. [Google Scholar]
- 50.Reimers CE, Jahnke RA, Mccorkle DC. Carbon fluxes and burial rates over the continental slope and rise off central California with implications for the global carbon cycle. Glob Biogeochem Cycles. 1992;6:199–224. [Google Scholar]
- 51.Joye SB, Anderson IC. Nitrogen cycling in coastal sediments. In: Capone DG, Bronk DA, Mulholland MR, Carpenter EJ, editors. Nitrogen in the Marine Environment. 2nd Ed. Academic; San Diego: 2008. pp. 867–915. [Google Scholar]
- 52.Devol AH. Denitrification, anammox, and N2 production in marine sediments. Annu Rev Mar Sci. 2015;7:403–423. doi: 10.1146/annurev-marine-010213-135040. [DOI] [PubMed] [Google Scholar]
- 53.Galbraith ED, et al. The acceleration of oceanic denitrification during deglacial warming. Nat Geosci. 2013;6:579–584. [Google Scholar]
- 54.Deutsch C, Sigman DM, Thunell RC, Meckler AN, Haug GH. Isotopic constraints on glacial/interglacial changes in the oceanic nitrogen budget. Glob Biogeochem Cycles. 2004;18:GB4012. [Google Scholar]
- 55.Brandes JA, Devol AH. A global marine-fixed nitrogen isotopic budget: Implications for Holocene nitrogen cycling. Glob Biogeochem Cycles. 2002;16:1120. [Google Scholar]
- 56.Gruber N. 2004. The dynamics of the marine nitrogen cycle and its influence on atmospheric CO2 variations. The Ocean Carbon Cycle and Climate, NATO Science Series, eds Follows M, Oguz T, (Springer, Dordrecht, The Netherlands), Vol 40, pp 97−148.
- 57.Ruttenberg KC. Reassessment of the oceanic residence time of phosphorus. Chem Geol. 1993;107:405–409. [Google Scholar]
- 58.Pyenson ND, Lindberg DR. What happened to gray whales during the Pleistocene? The ecological impact of sea-level change on benthic feeding areas in the North Pacific Ocean. PLoS One. 2011;6:e21295. doi: 10.1371/journal.pone.0021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braman RS, Hendrix SA. Nanogram nitrite and nitrate determination in environmental and biological materials by vanadium (III) reduction with chemiluminescence detection. Anal Chem. 1989;61:2715–2718. doi: 10.1021/ac00199a007. [DOI] [PubMed] [Google Scholar]
- 60.Sigman DM, et al. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal Chem. 2001;73:4145–4153. doi: 10.1021/ac010088e. [DOI] [PubMed] [Google Scholar]
- 61.Casciotti KL, Sigman DM, Hastings MG, Böhlke JK, Hilkert A. Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal Chem. 2002;74:4905–4912. doi: 10.1021/ac020113w. [DOI] [PubMed] [Google Scholar]
- 62.Breitenbach SFM, Bernasconi SM. Carbon and oxygen isotope analysis of small carbonate samples (20 to 100 µg) with a GasBench II preparation device. Rapid Commun Mass Spectrom. 2011;25:1910–1914. doi: 10.1002/rcm.5052. [DOI] [PubMed] [Google Scholar]
- 63.Yokoyama Y, Lambeck K, De Deckker P, Johnston P, Fifield LK. Timing of the Last Glacial Maximum from observed sea-level minima. Nature. 2000;406:713–716. doi: 10.1038/35021035. [DOI] [PubMed] [Google Scholar]
- 64.Wei K-Y, Chiu T-C, Chen Y-G. Toward establishing a maritime proxy record of the East Asian summer monsoons for the late Quaternary. Mar Geol. 2003;201:67–79. [Google Scholar]
- 65.Robinson RS, Etourneau J, Martinez PM, Schneider R. Expansion of pelagic denitrification during early Pleistocene cooling. Earth Planet Sci Lett. 2014;389:52–61. [Google Scholar]
- 66.Higginson MJ, Altabet MA, Murray DW, Murray RW, Herbert TD. Geochemical evidence for abrupt changes in relative strength of the Arabian monsoons during a stadial/interstadial climate transition. Geochim Cosmochim Acta. 2004;68:3807–3826. [Google Scholar]
- 67.Guo ZT, Berger A, Yin QZ, Qin L. Strong asymmetry of hemispheric climates during MIS-13 inferred from correlating China loess and Antarctica ice records. Clim Past. 2009;5:21–31. [Google Scholar]
- 68.Grinsted A, Moore JC, Jevrejeva S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process Geophys. 2004;11:561–566. [Google Scholar]
- 69.Hoering TC, Ford HT. The isotope effect in the fixation of nitrogen by azotobacter. J Am Chem Soc. 1960;82:376–378. [Google Scholar]
- 70.Minagawa M, Wada E. Nitrogen isotope ratios of red tide organisms in the East China Sea: A characterization of biological nitrogen fixation. Mar Chem. 1986;19:245–259. [Google Scholar]
- 71.Carpenter EJ, Harvey HR, Fry B, Capone DG. Biogeochemical tracers of the marine cyanobacterium Trichodesmium. Deep Sea Res Part I Oceanogr Res Pap. 1997;44:27–38. [Google Scholar]
- 72.Broecker WS, Patzert WC, Toggweiler JR, Stuiver M. Hydrography, chemistry, and radioisotopes in the Southeast Asian basins. J Geophys Res Oceans. 1986;91:2156–2202. [Google Scholar]
- 73.Amante C, Eakins BW. 2009. ETOPO1 1 Arc-Minute Global Relief Model: Procedures, Data Sources and Analysis (Natl Geophys Data Cent, Boulder, CO), NOAA Tech Memo NESDIS NGDC-24.
- 74.Lisiecki LE, Raymo ME. A Pliocene-Pleistocene stack of 57 globally distributed benthic δ18O records. Paleoceanography. 2005;20:PA1003. [Google Scholar]
- 75.Shakun JD, Lea DW, Lisiecki LE, Raymo ME. An 800-kyr record of global surface ocean δ18O and implications for ice volume-temperature coupling. Earth Planet Sci Lett. 2015;426:58–68. [Google Scholar]
- 76.Rohling EJ, et al. Sea-level and deep-sea-temperature variability over the past 5.3 million years. Nature. 2014;508:477–482. doi: 10.1038/nature13230. [DOI] [PubMed] [Google Scholar]
- 77.Sosdian S, Rosenthal Y. Deep-sea temperature and ice volume changes across the Pliocene-Pleistocene climate transitions. Science. 2009;325:306–310. doi: 10.1126/science.1169938. [DOI] [PubMed] [Google Scholar]