Significance
The understanding of the molecular mechanisms of activation and checkpoint processes has important therapeutic implications. Here, we show that interferon-γ is a master checkpoint regulator for many cytokines. It operates partially by activating STAT1 signaling. However, most important is the mechanism that allows it to assume master regulator status. To do this, it induces internalization of gp130, a common component of many heterodimeric cytokine receptors. Therefore, this cytokine checkpoint could open a whole new paradigm in cell biology.
Keywords: interferon-γ, cytokine, checkpoint, master regulator, differentiation
Abstract
Cytokines are protein mediators that are known to be involved in many biological processes, including cell growth, survival, inflammation, and development. To study their regulation, we generated a library of 209 different cytokines. This was used in a combinatorial format to study the effects of cytokines on each other, with particular reference to the control of differentiation. This study showed that IFN-γ is a master checkpoint regulator for many cytokines. It operates via an autocrine mechanism to elevate STAT1 and induce internalization of gp130, a common component of many heterodimeric cytokine receptors. This targeting of a receptor subunit that is common to all members of an otherwise diverse family solves the problem of how a master regulator can control so many diverse receptors. When one adds an autocrine mechanism, fine control at the level of individual cells is achieved.
As our understanding of physiology grows, we are increasingly aware of its Newtonian aspects in that for every action there is another reaction with opposite consequences (1). Irrespective of whether one thinks in terms of homeostasis, feedback loops, or checkpoints, we increasingly uncover molecular systems that, in terms of function, induce cells to move in opposite ways. For example, although immune T-cell activation is critical in controlling disease, one needs a checkpoint to guard against overactivity that could result in autoimmunity (2, 3).
In addition to adding to our general knowledge of cellular physiology, detailed understanding of the molecular mechanisms of activation and checkpoint processes has important therapeutic implications. Such an understanding allows two separate entry points into the regulation of cellular events. Thus, if one wants to promote a cellular function, the same outcome can be achieved by either enhancing the effector or inhibiting the checkpoint pathways, usually by perturbing the molecules that initiate them.
One of the most important physiological systems is the cytokine cascade (4). However, here, because the cascade contains large numbers of separate molecules, each operating through different receptors, simple models of regulation break down. While one can imagine that each cytokine is paired with a separate checkpoint system operating in an opposite direction, it seemed to us to be unlikely, if for no other reason than the economical use of genetic information. Given that one had large sets of related molecules with overlapping signal transduction mechanisms and pathways, it seemed more reasonable to assume that, depending on the circumstance, some members of the cytokine repertoire regulate other members of the repertoire. This differs from situations such as PD-1, where the checkpoint mechanistic cascade differs completely from the activation mechanism. By contrast, we propose that cytokine members of a family regulate each other by perturbing common molecular mechanisms. Thus, if gene action economy is proposed for a family, its hallmark should be that some of the same molecules used for signal transduction are differentially perturbed by different members of the family to achieve either activation or deactivation.
If these ideas are correct, the main experimental issue concerns how one can systematically discover pairs or higher order collections of molecules that constitute a system with both effector and opposing regulatory functions. To study this, we took a combinatorial approach to the problem similar to that used for antibody libraries (5, 6). We prepared a library in lentiviruses of the genes encoding 209 cytokines. The central idea was to first select cells expressing a cytokine that induces a phenotype and then challenge these cells with other cytokines to determine if any interfered with the induction process. In other words, we used a combinatorial selection process to find pairs of molecules that function robustly in opposite directions. We found several cytokines in which the signal transduction pathway leading to odontoblastic differentiation in dental pulp stem cells (DPSCs) was completely shut down by IFN-γ (IFNG). The unique features of this checkpoint involve two independent mechanisms. First, IFNG decreased cytokine-induced activation of STAT3 while increasing STAT1 signaling. STAT1 may compete for STAT binding sites on the receptor, thereby interfering with activation of STAT3. Second, IFNG induced internalization of the common cytokine receptor gp130 by activating p38 signaling. The process appears to be general because IFNG inhibits the function of multiple cytokines belonging to bone morphogenetic proteins (BMPs) and the interleukin 6 (IL-6) family of cytokines. IFNG can function as a master switch because it targets the common subunit of an otherwise diverse set of these heterodimeric receptors. In summary, we report on a combinatorial method to select for members of a pathway that perturb the activity of other members of the same pathway.
Selecting a Cytokine System with Robust On/Off Switches
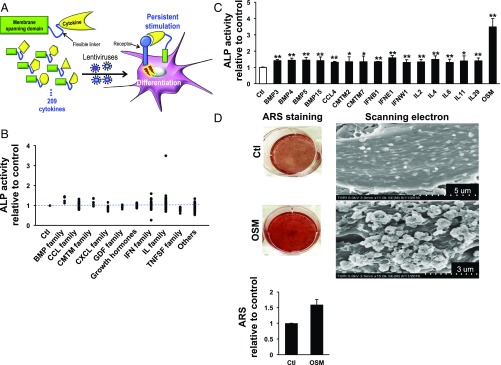
The major hypothesis of this study is that cytokines are checkpoint regulators of each other. Thus, this investigation requires selection of cytokine pairs or higher order combinations of molecules that either activate or inactivate a phenotype. We began with the assumption that such on/off signals could be seen for pairs of cytokines. To isolate such pairs, we established a human cytokine library in lentiviruses containing 209 different human cytokines. To enhance the efficacy of these cytokines, we used a display format in which cytokines are tethered to the plasma membranes of target cells (Fig. 1A). When membrane-bound cytokines are used, persistent stimulation by the cytokine is expected because receptor-mediated endocytosis is reduced and the effective molarity for target receptors is enhanced (5). To verify whether cytokines in this library format behave as expected, we tested them in two cell models, U937 monocytes and HEK293-NF-κB-GFP cells (Fig. S1). When the known cytotoxic cytokine TNF-α was expressed in U937 monocytes, it strongly increased cell death (Fig. S1) (7). Moreover, TNF-α and IL-1β both potently activated NF-κB signaling, represented by an increase in NF-κB–induced GFP expression in HEK293-NF-κB-GFP cells (Fig. S1) (8, 9). With this validation in hand, we selected human DPSCs as a model system because, as multipotent stem cells, they have the potential to differentiate into a variety of cell types (10–13). In this way, the diversity of potential phenotypes somewhat matches the input diversity of the cytokine library. Initially, we studied the general problem of osteogenesis, largely because one can get a quantitative differentiation by measuring the amount of enzymes and mineral depositions specific to bone formation. But, because the primary cell is a dental stem cell, we refined our search to the more specific problem of induction of odontoblasts. To probe the role of cytokines in induction of differentiation, we infected DPSCs with the cytokine library and then measured differentiation by using a quantitative alkaline phosphatase (ALP) assay (Fig. 1B). There were 16 positive inducers of odontoblastic differentiation in the library, characterized by a significant increase in ALP activity (Fig. 1C). In addition to well-known osteogenic inducers such as BMPs, several IL-6 family cytokines, including IL-6, IL-11, and Oncostatin M (OSM), showed significant effects on differentiation (14–16). Most of the known positive regulators enhanced odontoblastic differentiation only slightly (∼1.5-fold), but OSM increased differentiation over three times, as measured by ALP assay (Fig. 1C). In addition to ALP assay, Alizarin Red S (ARS), an anthraquinone derivative, staining also has been widely used to evaluate calcium deposit in bone cells. Using ARS staining and scanning electron microscopy analysis, we observed that OSM-induced odontoblastic differentiation was accompanied by abundant mineral deposits on the cell surface (Fig. 1D and Fig. S2).
Fig. 1.
Selection of potent cytokine switches through unbiased screening of an infectious human cytokine library. (A) Scheme representing the selection of active cytokines that regulate the differentiation of DPSCs from autocrine-based human cytokine libraries; 209 cytokines were screened in the human cytokine library. Each cytokine was attached to the transmembrane domain of platelet-derived growth factor receptor via a flexible linker and then incorporated into lentiviruses used to infect DPSCs. (B) Odontoblastic differentiation of DPSCs was measured by ALP assay 10 d after infection with the lentiviral cytokine library. The mean values of ALP activity from two independent experiments are shown. Dots represent individual members of the cytokine families indicated on the x axis. Ctl represents DPSCs cultured in basal osteoblastic differentiation media. (C) Sixteen cytokines from the cytokine library were identified as positive inducers of odontoblastic differentiation based on increased ALP activity. OSM most strongly increased ALP activity. Data are shown as mean + SD (n = 6). (D) ARS staining and scanning electron microscopy of DPSCs 10 d after incubation with OSM confirmed that OSM most strongly induced DPSC differentiation compared with Ctl. The quantification of ARS staining was performed twice and the mean values are shown. *P < 0.05, **P < 0.01.
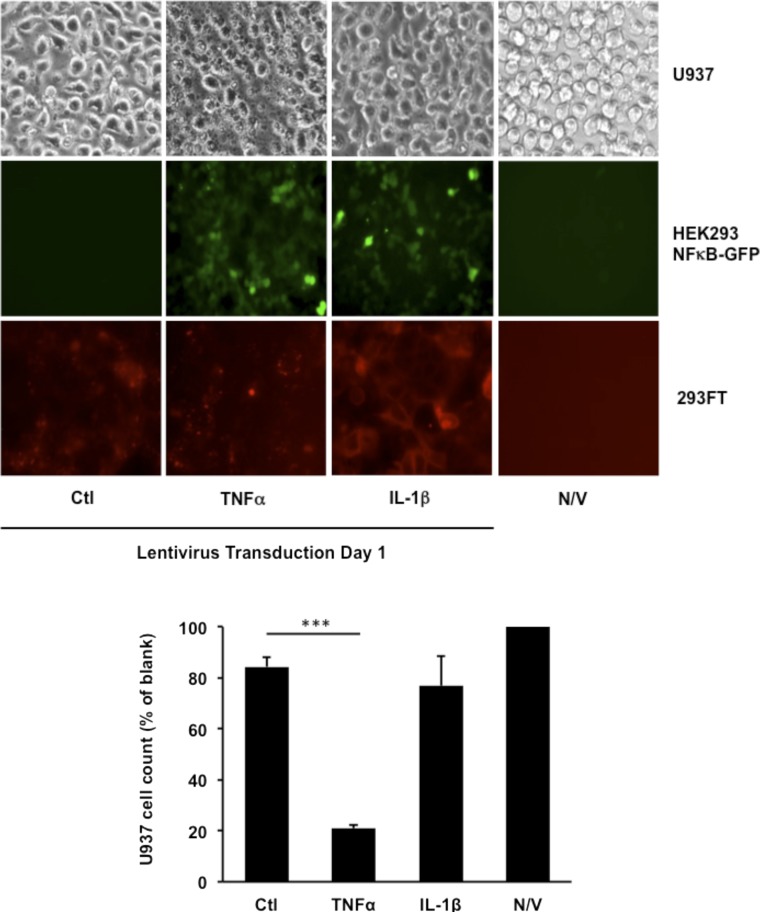
Fig. S1.
Validation of the infectious human cytokine library. Lentiviruses containing control vector, IL-1β, or TNF-α were transduced to U937 monocytes, HEK293 cells with fluorescent reporter gene (NF-κB-GFP), or 293FT cells for 1 d. Light microscopy was used to record cell morphology as an indicator for cell survival of U937 monocytes following lentivirus transduction. The quantification of cell number was performed with bright-field pictures from three independent experiments by using the Cell Counter plugin of ImageJ (https://imagej.nih.gov/ij/plugins/cell-counter.html). HEK293-NF-κB-GFP cells increased GFP (green) expression in response to TNF-α and IL-1β lentivirus treatment, as shown by fluorescence microscopy. Expression of the cytokine constructs was verified by the mCherry (red) fluorescence in infected 293FT cells. Data are shown as mean + SD (n = 3). ***P < 0.001. N/V, no virus control.
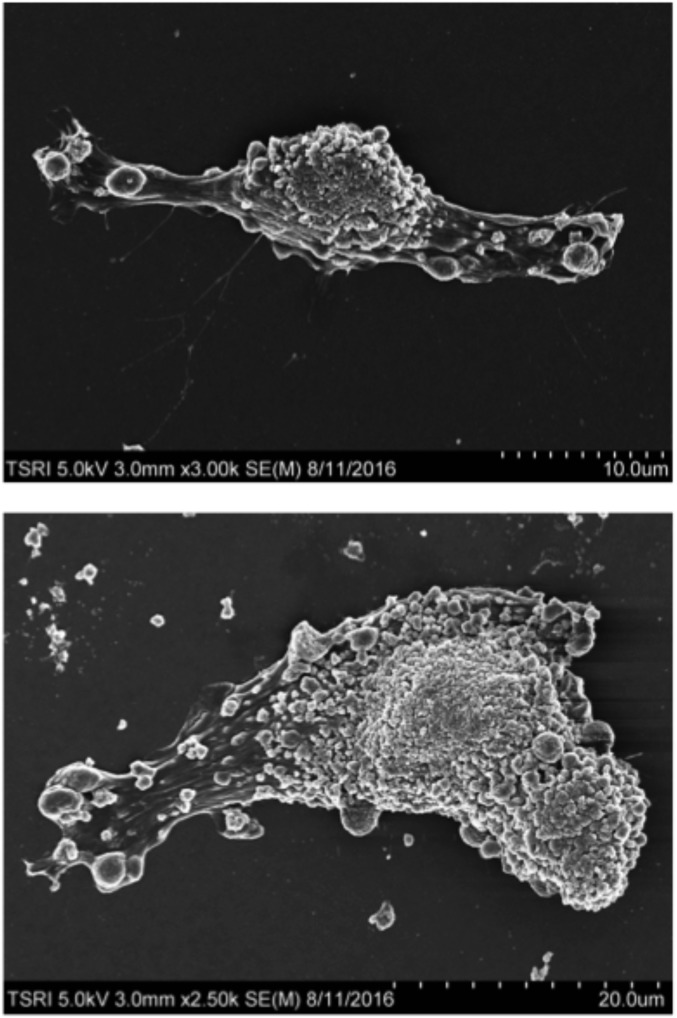
Fig. S2.
Scanning electron microscopy analysis of odotoblastic differentiation by OSM. Scanning electron microscopy analysis of DPSCs after incubation with OSM for 10 d. These panels show representative images of fully differentiated DPSCs by OSM.
IFNG Is a Potent Checkpoint Switch for the Cytokine-Driven Odontoblastic Differentiation
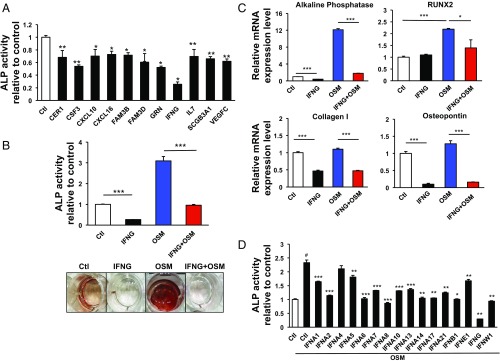
In addition to the 16 cytokines that enhanced the odontoblastic differentiation, there were 11 cytokines that showed opposite activities (Figs. 1B and 2A). IFNG had by far the most potent inhibitory effect on odontoblastic differentiation (Fig. 2A and Fig. S3). We wondered whether the potent inhibition by IFNG under basal conditions also functioned in the presence of a strong activator of differentiation such as OSM. When DPSCs were cotreated with IFNG and OSM together, the OSM-induced ALP activity was strongly inhibited by IFNG (Fig. 2B). Similarly, the OSM-induced transcriptional regulation of osteogenic genes including ALP, Runt-related transcription factor 2 (RUNX2), Collagen I, and Osteopontin was also significantly decreased by IFNG treatment (Fig. 2C) (17–19).
Fig. 2.
Identification of IFNG as a potent negative regulator for cytokine-induced differentiation of DPSCs. (A) Out of 11 identified negative regulators, IFNG most strongly inhibited odontoblastic differentiation based on ALP assay results. *P < 0.05, **P < 0.01. Data are shown as mean ± SD (n = 6). (B) ALP assays and ARS staining were performed to evaluate the effect of IFNG on DPSC differentiation under stimulation with OSM 10 d after lentivirus infection. IFNG prevented OSM-induced increases in ALP activity and ARS staining. ***P < 0.001. (C) qPCR analysis was used to determine mRNA levels of ALP, RUNX2, Collagen I, and Osteopontin, typical markers for DPSC differentiation. IFNG prevented OSM-induced up-regulation of all four markers. *P < 0.05, ***P < 0.001. (D) Several subtypes of IFN lentiviruses were tested for the effect on ALP activity in DPSCs under OSM stimulation 10 d after infection. *P < 0.05, **P < 0.01, ***P < 0.001 (comparison between OSM only vs. individual cytokines); #P < 0.001 [control (Ctl) vs. OSM only]. Data are shown as mean + SD (n = 3) in B to D.
Fig. S3.
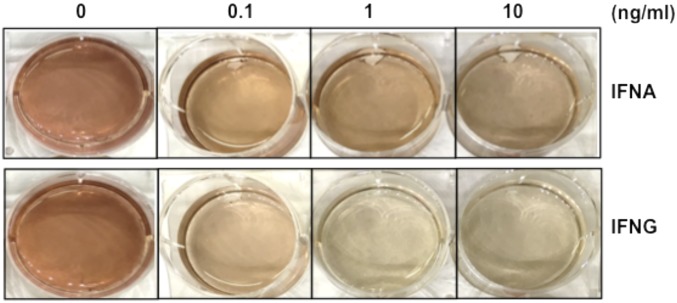
Dose-dependent regulation of the odontoblastic differentiation by cytokine IFNA and IFNG proteins were proteins incubated with DPSCs at several concentrations, namely 0, 0.1, 1, or 10 ng/mL, for 10 d. These cells were stained with ARS.
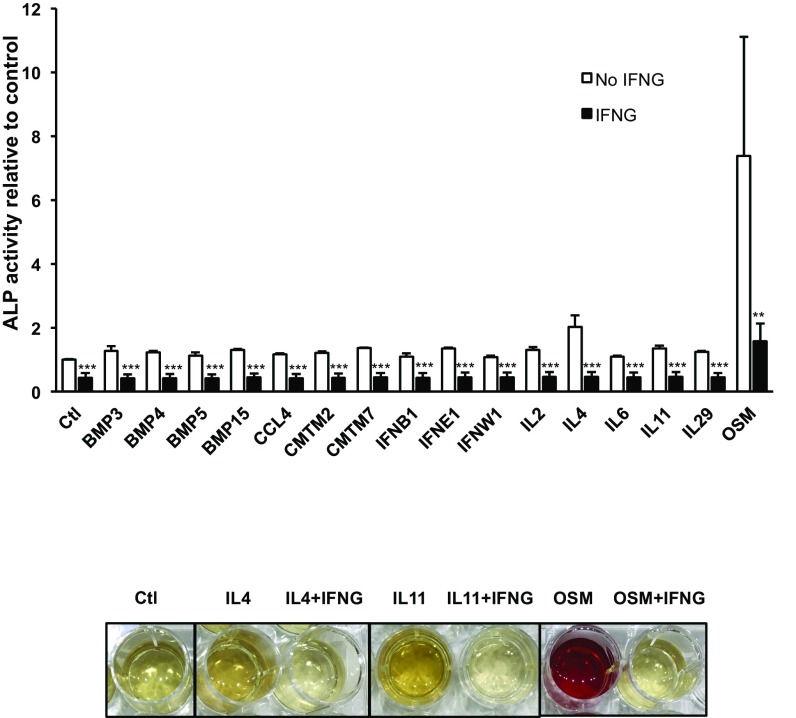
Since interferons are a group of different cytokines with a wide array of effects (20), we next investigated whether the inhibitory effect of IFNG on odontoblastic differentiation is specific to the type II IFN subtype. Different subtypes of IFN were compared with IFNG regarding their ability to inhibit OSM-induced odontoblastic differentiation. Although several type I IFNs also showed some blocking effect, their potency was much lower than IFNG (Fig. 2D). Finally, we tested whether IFNG was a “master regulator” in that it could modulate other cytokines involved in odontoblastic differentiation. In addition to its effects on OSM-induced differentiation, IFNG down-regulated the induction of differentiation by all of the other cytokines tested in the ALP assay (Fig. 3, Top). We also observed in the ARS assay that the cytokine-induced differentiation was strongly inhibited by IFNG (Fig. 3, Bottom). These data suggest that IFNG may be a master checkpoint regulator of many cytokines.
Fig. 3.
IFNG is a master regulator of cytokine-driven odontoblastic differentiation. DPSCs were coinfected with IFNG lentivirus and a lentivirus containing one of the 16 differentiation-inducing cytokines (Top). IFNG inhibited an increase in ALP activity induced by any of those differentiation-inducing cytokines. IFNG also suppressed the increase of ARS staining induced by cytokines (Bottom). Data are shown as mean + SD (n = 6). **P < 0.01, ***P < 0.001.
Checkpoint Mechanisms
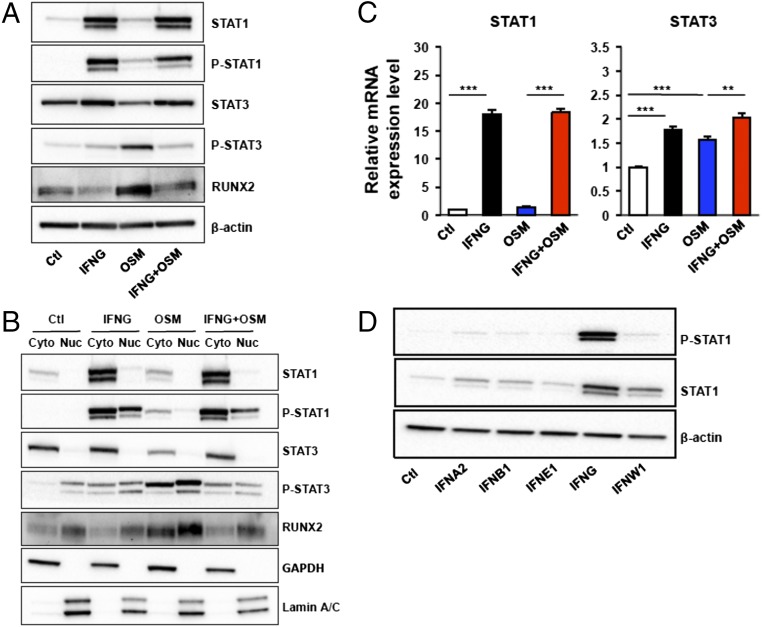
Because STAT signaling is commonly used by many cytokine families, we began our studies on mechanism by studying the effect of IFNG on their expression and activation (21, 22). We studied STAT signaling after OSM treatment by measuring STAT protein levels and their degree of phosphorylation in differentiating DPSCs. OSM strongly elevated phosphorylation of STAT3 while STAT1 and phospho (P)-STAT1 levels remained unaltered (Fig. 4A). In contrast, IFNG potently elevated phosphorylation of STAT1. Surprisingly, in addition to the activation of STAT1 phosphorylation by IFNG, there was potent up-regulation of its absolute amount (Fig. 4 A and B). To study if expression of STAT genes was influenced by cytokines, we analyzed the mRNA level of STAT1. As with the protein levels of STAT1, the mRNA level of STAT1 was strongly (∼20-fold vs. control) increased by IFNG (Fig. 4C), confirming that IFNG induces STAT1 expression on a transcriptional level. OSM also potently induced RUNX2, a key transcription factor that is associated with osteoblast differentiation (Fig. 4 A and B). Interestingly, when OSM and IFNG were used together, IFNG significantly inhibited the phosphorylation of STAT3 and the level of RUNX2 (Fig. 4 A and B).
Fig. 4.
IFNG potently increased levels of STAT1 and P-STAT1 while preventing cytokine-induced phosphorylation of STAT3. (A) Western blot analysis was used to compare expression levels of STAT1, STAT3, and RUNX2 in DPSCs after treatment with IFNG virus, OSM virus, or both (IFNG plus OSM viruses). (B) The lysates in A were separated into cytosolic and nuclear fractions and then separately analyzed by Western blot. GAPDH and Lamin A/C served as internal controls for cytosolic and nuclear fractions. (C) mRNA levels of STAT1 and STAT3 were measured by qPCR after treatment of DPSCs with IFNG, OSM, or IFNG plus OSM. Data are shown as mean + SD (n = 3). (D) STAT1 and P-STAT1 levels in DPSCs were compared after incubation with lentiviruses containing different IFN subtypes. IFNG most strongly induced STAT1 and P-STAT1 expression. **P < 0.01, ***P < 0.001.
The STAT proteins are known to translocate into the nucleus after phosphorylation (23). Thus, we analyzed the level of P-STAT3 both in the cytosol and nucleus after cytokine treatment. Intriguingly, the ratio (nucleus/cytosol) of P-STAT3 induced by OSM was also decreased by IFNG treatment (Fig. 4B). To determine if the observed up-regulation of STAT1 is specific to IFNG, we measured the STAT1 protein level after treatment of cells with different subtypes of IFNs. In contrast to IFNG, other subtypes only mildly increased the STAT1 level compared with control (Fig. 4D). Collectively, these data indicate that IFNG specifically induced the inhibition of STAT3 phosphorylation and sequestration of transcriptionally active P-STAT3.
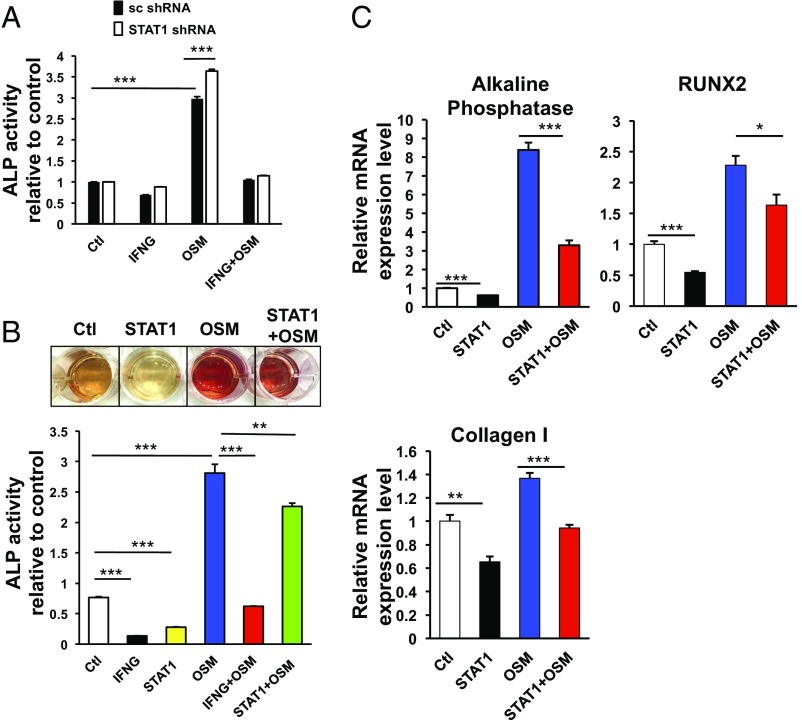
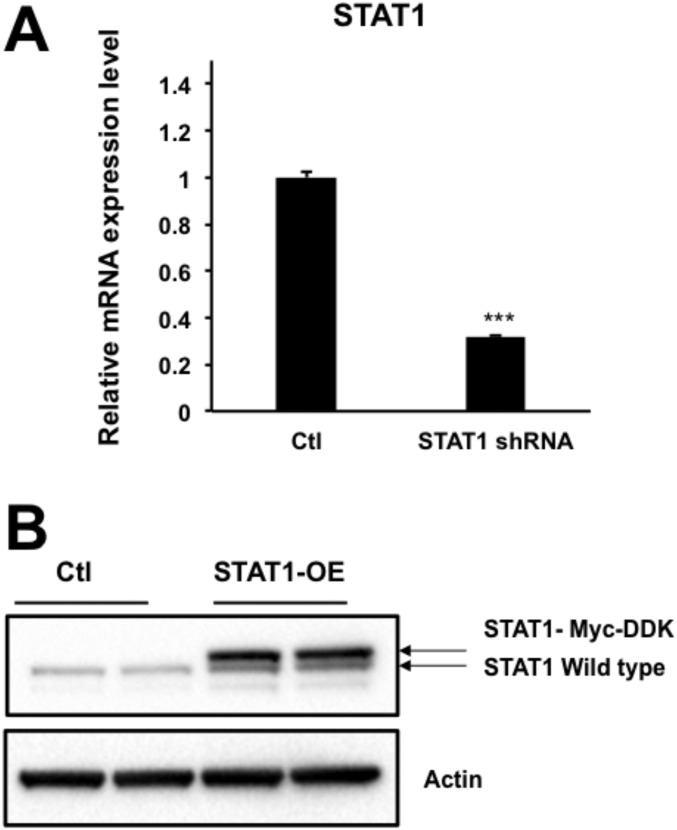
It has been reported that different types of STAT proteins can antagonize each other, for example by blocking the formation of active complexes for transcription (24). We thus hypothesized that the observed up-regulation of STAT1 by IFNG may interfere with the STAT3-dependent odontoblastic differentiation. To verify our hypothesis, we analyzed the checkpoint regulation of IFNG in DPSCs after knockdown (KD) of STAT1. Unexpectedly, the STAT1 KD did not affect the suppressive effect of IFNG on OSM-activated odontoblastic differentiation of DPSCs (Fig. 5A and Fig. S4A).
Fig. 5.
STAT1 is not a major mediator for the observed inhibitory effect of IFNG on cytokine-driven odontoblastic differentiation. (A) KD of STAT1 does not abolish the inhibitory effect of IFNG on OSM-induced DPSC differentiation measured by ALP activity. DPSCs were preinfected with lentiviruses of scrambled (sc) shRNA or STAT1 shRNA and subsequently transduced with lentiviruses containing control vector, IFNG, OSM, or IFNG plus OSM. After 6 d, ALP activity was measured. (B and C) Overexpression of STAT1 does not prevent OSM-induced DPSC differentiation as potently as IFNG. DPSCs were incubated with lentiviruses containing control vector, IFNG, STAT1, OSM, IFNG plus OSM, or STAT1 plus OSM, followed by ALP assay, ARS staining (B), or qPCR analysis to determine mRNA levels of ALP, RUNX2, and Collagen I (C). Data are shown as mean + SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. S4.
KD or overexpression (OE) of STAT1. Lentiviruses containing STAT1 shRNA or STAT1 gene for loss- or gain-of-function studies were constructed and validated by qPCR (A) or Western blot (B) analysis. Data are shown as mean + SD (n = 3). ***P < 0.001.
In addition to the loss-of-function study of STAT1, we also carried out gain-of-function studies regarding the role of STAT1 in mediating the checkpoint function of IFNG by overexpressing STAT1. First, we overexpressed STAT1 in DPSCs and analyzed whether elevated STAT1 affected the differentiation of DPSCs. STAT1 overexpression moderately suppressed odontoblast differentiation from DPSCs (Fig. 5B and Fig. S4B). Also, OSM-mediated expression of its target genes such as ALP, RUNX2, and Collagen I was partially decreased (Fig. 5C). However, the effects observed after overexpression of STAT1 are moderate relative to those seen after IFNG treatment.
Together, these studies suggest that STAT1 is involved in checkpoint regulation by IFNG. However, while perturbation of STAT1 levels may partially account for the observed decrease in cytokine-induced differentiation by IFNG, it alone cannot account for the massive effect of IFNG on OSM. Thus, we searched for additional components of the mechanism. We focused on the OSM receptor (OSMR) complex that, as for some other cytokine receptors, is a heterodimer consisting of a common gp130 subunit and its own receptor, OSMR (25). Importantly, gp130 represents an important receptor component for many cytokines, including IL-6, IL-11, IL-27, OSM, and leukemia inhibitory factor (26). Indeed, we showed (see above) that several receptors such as OSM, IL-6, and IL-11 that were able to induce odontoblastic differentiation all contained the gp130 subunit.
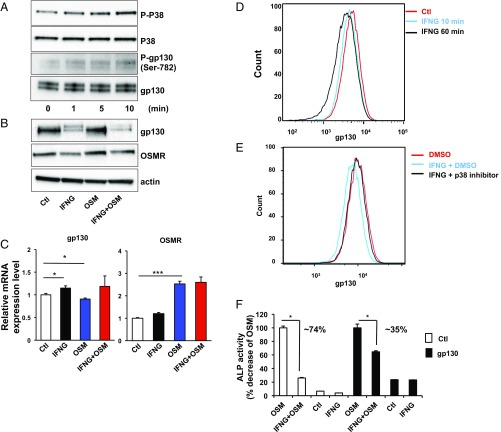
There have been several reports that gp130 internalizes after ligand binding (27). This occurs after activation via a p38-mediated pathway during which activated p38 phosphorylates the Ser-782 residue of gp130 before its internalization (28, 29). Thus, we first tested whether IFNG affects p38 phosphorylation in DPSCs. When the DPSCs were acutely treated with recombinant IFNG, phosphorylation of p38 and gp130 rapidly increased in a time-dependent fashion (Fig. 6A). Next, we measured the amount of gp130 protein after long-term treatment of DPSCs with IFNG (Fig. 6B). Interestingly, the gp130 protein level was significantly decreased by IFNG treatment. In contrast, the OSMR, the non-gp130 component of the heterodimeric receptor complex, was not decreased at the protein or mRNA level by IFNG treatment (Fig. 6 B and C). Thus, cytokine receptors consist of heterodimers with one shared and one nonshared subunit, and IFNG only down-regulates the shared subunit. The fact that IFNG operates by modulating a component shared by many cytokine receptors may explain how it functions as a master regulator.
Fig. 6.
IFNG induces internalization of gp130 in a p38-dependent way. (A) Western blot analysis showed that treatment of DPSCs with recombinant IFNG for 0, 1, 5, or 10 min increased phosphorylation levels of p38 and gp130. (B and C) Protein (B) and mRNA (C) levels of gp130 and OSMR were analyzed 6 d after infection of DPSCs with lentiviruses containing control vector, IFNG, OSM, or IFNG plus OSM. (D and E) FACS analysis showed that treatment with DMSO or recombinant IFNG for 10 or 60 min decreased gp130 antibody staining on the cell surface of DPSCs. Pretreatment with a p38 inhibitor for 1 h prevented those changes. (F) Preincubation with gp130 lentivirus for 1 d resulted in a 35% decrease in ALP activity in IFNG plus OSM–treated DPSCs compared with OSM-treated cells, while the difference in ALP activity between OSM and IFNG plus OSM–treated DPSCs following preincubation with control lentivirus was 74%. Data are shown as mean + SD (n = 3). *P < 0.05, ***P < 0.001.
Finally, we tested whether the gp130 component of the receptor complex on the cell surface is lost through IFNG-induced internalization. To study this, we performed a fluorescence-activated cell sorting (FACS)-based receptor internalization assay after IFNG treatment of DPSCs. To label the gp130 proteins on a cell surface, we stained them with a fluorescent anti-gp130 antibody. After treatment with IFNG for 1 h, the level of gp130 on the cell surface was decreased by 30% (Fig. 6D). The original gp130 level was restored when the cells were treated by a p38-specific inhibitor (Fig. 6E). Furthermore, the suppressive effect of IFNG on OSM-induced osteogenesis was drastically reversed by overexpressing gp130 (Fig. 6F). In toto, these data demonstrate that IFNG functions as a checkpoint regulator for cytokine-induced odontoblastic differentiation by modulating the two independent STAT1 and p38 components of signal transduction cascades.
The Regulation Is Likely Autocrine
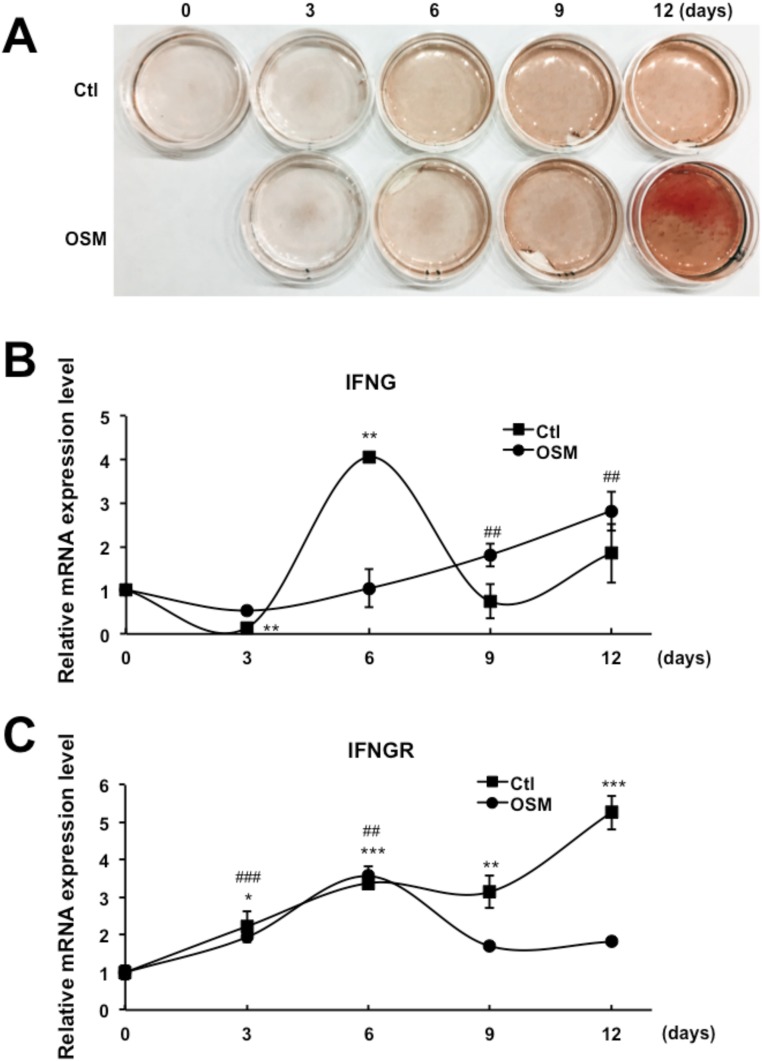
The operating premise of this study is that checkpoint control of cytokine function is most efficient if members of the family regulate each other. The system could be further optimized if an autocrine mechanism is used because regulation would be directly linked to differentiation in the cell where it is occurring. Thus, if IFNG is a critical checkpoint regulator for odontoblastic differentiation, it is likely that expression of it and its receptor can occur in the same cell so that the checkpoint activity relates directly to the differentiation status of the cell. To address this question, we first measured the mRNA levels of IFNG and the IFNG receptor in the presence of basal differentiation media over a 12-d period (Fig. S5). Intriguingly, in the absence of OSM, the IFNG level early in the process of differentiation (day 3) was significantly reduced and rebounded by 6 d as differentiation increased (Fig. S5B). However, in the presence of OSM, the IFNG level was slightly reduced at day 3 and remained depressed until day 6 (Fig. S5B). These results suggest that the expression of the IFNG checkpoint is dampened while the differentiation cascade is proceeding. Later, a negative feedback loop is engaged to regulate differentiation. In addition to IFNG, we also analyzed the mRNA level encoding its receptor (Fig. S5C). Similar to IFNG, its receptor increases during differentiation, likely functioning as a component of the negative feedback loop. This elevation of IFNG receptor expression may sensitize the responsiveness of cells against both autocrine and immune cell-generated IFNG.
Fig. S5.
IFNG checkpoint is controlled as an autocrine system. DPSCs were treated with lentiviruses containing control vector or OSM. (A) ARS staining shows odontoblastic differentiation over the time course of 12 d with or without OSM. (B and C) RNA samples were collected on days 0, 3, 6, 9, and 12 to compare mRNA levels of IFNG (B) and IFNG receptor (C) over time. Data are shown as mean + SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001, ##P < 0.01, ###P < 0.001.
Universality of Cytokine Checkpoint Regulation
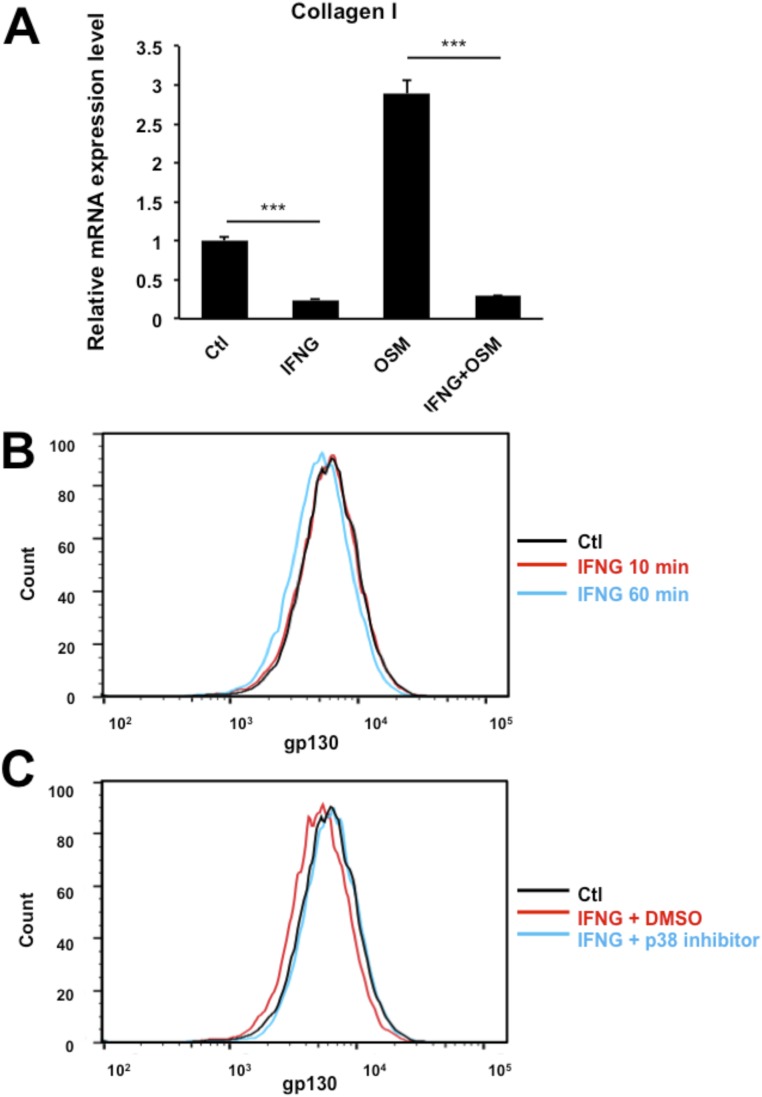
To show that IFNG is a master checkpoint regulator in multiple biological processes, we tested to see if it can influence a cytokine-induced cellular process, which is completely irrelevant to stem cell differentiation. Several reports have suggested that OSM can modulate the extracellular matrix such as collagen in various fibroblasts (30, 31). When the primary human lung fibroblasts were treated with OSM, it enhanced collagen I expression (Fig. S6A). When human lung fibroblasts were cotreated with IFNG and OSM simultaneously, the OSM-induced collagen I synthesis was completely blocked (Fig. S6A). Furthermore, IFNG induced the internalization of gp130 in human lung fibroblasts p38-dependently (Fig. S6 B and C). Hence, IFNG may play a role as a master cytokine checkpoint regulator in general cellular processes, not only in the differentiation of DPSCs. Finally, the regulation of collagen synthesis may be relevant to a variety of diseases, including pulmonary fibrosis and keloid formation.
Fig. S6.
IFNG inhibits OSM-induced Collagen I production in human lung fibroblasts (HLFs). (A) HLFs were infected with IFNG and OSM lentiviruses alone or in combination. Collagen I mRNA level was analyzed by real-time PCR 3 d after infection. Data are shown as mean + SD (n = 3). (B and C) FACS analysis shows that treatment with DMSO or recombinant IFNG for 10 or 60 min decreased gp130 antibody staining on the cell surface of HLFs. Pretreatment with a p38 inhibitor for 1 h prevented those changes. ***P < 0.001.
Discussion
The main question addressed in this study concerns how sets of molecules with overlapping mechanisms and pathways are regulated. This could occur by extrinsic extracellular or intrinsic intracellular mechanisms. In intrinsic mechanisms, members of the set regulate each other, whereas for extrinsic mechanisms, proteins orthogonal to the set are used as regulatory molecules. In this study, we show that the OSM checkpoint appears to use an intrinsic mechanism based on the ability of IFNG to modulate the receptor. Although this is only one system, it makes general sense. There are ≈209 known cytokines. If they were extrinsically regulated there would have to be hundreds of such systems. Indeed, extrinsic regulation of such a large system would compromise the gene economy of the organism.
IFNG Is a Primary Checkpoint for Cytokine-Induced Odontoblastic Differentiation of DPSCs.
DPSCs are a type of mesenchymal stem cell (MSC), which have self-renewal and multilineage differentiation potential, including formation of osteoblasts, adipocytes, chondrocytes, muscle cells, and neural cells (12, 13, 32, 33). This multipotency of DPSCs is critical for dental pulp repair and regeneration of teeth. Hence, understanding their differentiation regulation is important for future studies such as applications in regenerative medicine. Herein, we found that several cytokine families, including the BMP family and IL-6 family cytokines, induced odontoblastic differentiation of DPSCs. Intriguingly, all of the cytokine-dependent odontoblastic differentiations of DPSCs appear to be potently blocked by the IFNG checkpoint, which governs the independent signaling pathways STAT1 and p38.
STAT1 Up-Regulation Interferes with Odontoblastic STAT3 Signaling of OSM.
In response to cytokines, STAT3 proteins are phosphorylated and form homo- or heterodimers, and translocate to the nucleus, where they act as transcription activators. The STAT3 signal pathway has been reported to be important for odontoblastic differentiation in MSCs by cytokines (34). We have observed that IFNG reduced the phosphorylation level of STAT3 and that this activity was STAT1-dependent. IFNG potently elevated both the expression and the phosphorylation of STAT1. There are several consequences resulting from STAT1 elevation that can perturb STAT3 signaling. First, the elevated STAT1 may compete with STAT3 for binding to receptor docking sites, the DNA-binding element, or cofactors. This may also lead to the sequestration of STAT3 from transcriptionally active STAT3 homodimers by inducing STAT1–STAT3 heterodimers. In addition to STAT3, STAT1 also can interfere with other types of STAT proteins as well. Therefore, the simple elevation of STAT1 by IFNG is an effective way to interfere with multiple cytokines using STATs as key signal transducers.
Initially, unphosphorylated STATs were considered to be nascent transcription activators in the cytoplasm, entering the nucleus to induce gene expression only after activation by phosphorylation. However, reports have proven that STAT1 and STAT3 are present in nuclei independent of phosphorylation (35–38). These unphosphorylated STAT1s have been found to drive some genes, such as the low-molecular mass polypeptide 2 gene, by forming the complex with IFN regulatory factor 1. Hence, in addition to the effect of elevated STAT1 as a competitor to other STAT functions, the orthogonal regulation by IFNG-induced up-regulation of unphosphorylated STAT1 may affect the checkpoint activity of IFNG in the cytokine-induced odontoblastic differentiation.
Solving the Problem of a Master Switch.
A master regulator must modulate diverse proteins. Thus, the problem reduces to the question of how a single protein modulates sets of diverse proteins that in our case are receptors. The problem is solved when the receptor is a multimeric complex containing both common and variable components. If the regulatory target is the common subunit of otherwise different receptors, they can all be regulated by a single protein. The gp130 component of cytokine receptors is a shared signaling subunit of numerous IL-6 family cytokines and is abundantly expressed in DPSCs (Fig. 6 A and B). In this study, we observed that several IL-6 family cytokines, including IL-6, IL-11, and OSM, shared this gp130 component in their signal-transducing receptors. IFNG potently reduced the level of gp130 by inducing its internalization in a p38-dependent manner. The IFNG-induced gp130 internalization perturbed both the basal and the OSM-induced odontoblastic differentiation. Interestingly, IFNG strongly reduced only gp130, which is the common component of the heterodimeric receptor, without affecting the level of the OSMR, which is the variable component. IFNG also down-regulated receptors that do not contain gp130. For these receptors, up-regulation of STAT1 may, in some cases, suffice as a checkpoint inhibitor.
Precise Linkage of Differentiation and Its Checkpoint.
The master regulation of differentiation by IFNG is autocrine-based. Thus, as differentiation proceeds, the amount of IFNG and its receptor increases. This mechanism allows the regulatory machinery of a cell to be precisely linked to the differentiation cascade. The regulatory machinery is off until differentiation reaches a certain state, after which it switches on. Arguably, this is the most precise way to regulate differentiation in organs because cells are not synchronous and, in an autocrine mode, each cell is a separately contained system.
Previous studies have reported that IFNG is involved in the regulation of the osteoblastic differentiation of bone marrow-derived MSCs (39). However, largely because there are few data on mechanism, the role of IFNG is unclear as to whether it induces or inhibits differentiation in these cells (39, 40). Now, with the data on the mechanism reported here, it is clear that IFNG is a potent checkpoint inhibitor of osteogenesis, and we can understand how it can function as a master regulator of differentiation for the many different stem cells that have IFNG receptors. The fact that IFNG is the only type II IFN and differs from other IFNs in structure and sequence is compatible with its unique role as a master regulator of differentiation.
Materials and Methods
Construction of the Lentiviral Cytokine Library.
Human cytokine gene cDNAs (209) were obtained from the GE cytokine library. PCR primers were designed for each cDNA to introduce a pair of SfiI sites that are compatible with the lentiviral vector pLV2-EF1a-MTA to the ends of each PCR-amplified insert. After digestion by SfiI, the inserts were ligated into the SfiI-digested lentiviral vector separately to construct 209 lentiviral cytokine plasmids.
See SI Materials and Methods for a detailed description.
Infection of DPSC with Lentivirus.
Lentivirus was added to DPSC in the DPSC growth medium containing 5 μg/mL polybrene and incubated at 37 °C, 5% CO2 for 24 h. Virus-containing medium was replaced with fresh osteogenesis induction medium, which contains the hMSC differentiation basal medium-osteogenic (Lonza) and the hMSC osteogenic SingleQuots kit (Lonza). Cells were fed with fresh osteogenesis induction medium every 3–4 d until ready for assay.
SI Materials and Methods
Antibodies.
Antibodies of anti-STAT1, P-STAT1, STAT3, P-STAT3, RUNX2, gp130, β-actin, GAPDH, Lamin A/C, p38, and P-p38 were from Cell Signaling. Anti-OSMR antibody was obtained from R&D. Anti–p-gp130 antibody was from Santa Cruz.
Packaging of Lentivirus.
HEK-293FT cells were cotransfected by lentiviral cytokine plasmids with the pCMVD8.9 and pVSVg viral packaging vectors at a ratio of 1:1:1. The DNA–lipid complex was removed after overnight incubation, and fresh medium was added to the cells. Supernatant containing virus was collected after 48 h and filtered through a 0.22-μm polyethersulfone membrane filter unit (Millipore). The virus titer was determined by using a Lenti-X p24 ELISA Kit (Clontech).
Cell Culture.
DPSCs were obtained from Lonza and cultured in DPSC growth medium, which includes DPSC basal medium (Lonza) and the DPSC SingleQuots Kit (Lonza). DPSCs from passages 2 to 4 were used for experiments.
ALP Assay.
After 6 to 7 d of osteogenesis induction, the ALP activity of DPSCs was determined by using an ALP Activity Colorimetric Assay Kit (BioVision). Briefly, cells were washed twice with cold Dulbecco's PBS (DPBS) and lysed in cell lysis buffer on ice for 10 min. Cell lysate was collected and centrifuged at 12,000 × g, 4 °C for 10 min to remove cell debris. The supernatant was aliquoted in triplicate to a 96-well plate. Following incubation with p-nitrophenylphosphate solution at 37 °C for 20 min, stop solution was added to end the reaction. The absorbance of each well at 405 nm was read by a Tecan Infinite 200 PRO plate reader.
ARS Staining.
After 12 to 14 d of osteogenesis induction, the extracellular calcium deposits of DPSCs were detected by ARS. Cells were washed with DPBS (without Ca2+ and Mg2+) and fixed by 4% (wt/vol) paraformaldehyde solution (Affymetrix) for 30 min at room temperature. After washing with distilled water, 2% (wt/vol) ARS staining solution (pH 4.1 to 4.3) was added to stain the cells in the dark for 45 min at room temperature. Then cells were carefully washed with distilled water three or four times and kept in DPBS for imaging. The quantification of ARS staining was determined by ARS Staining Quantification Assay (8678; ScienCell). After removing excess water from the stained plates, 10% acetic acid was added and incubated at room temperature for 30 min with gentle shaking. Samples were mixed by pipetting, transferred to 1.5-mL tubes, and heated at 85 °C for 10 min. Then the tubes were incubated on ice for 5 min and centrifuged at 20,000 × g for 15 min. The supernatants were transferred to new tubes, and 10% ammonium hydroxide was added to neutralize the acid. After aliquoting to a 96-well plate, the absorbance at 405 nm was read by a Tecan Infinite 200 PRO plate reader.
Real-Time PCR.
After 3 d of osteogenesis induction, total RNA was isolated from DPSCs using TRIzol Reagent (Life Technologies) following the manufacturer’s protocols. The cDNA was prepared by using an iScript cDNA Synthesis Kit (Bio-Rad). Real-time PCR was performed in triplicate on a Bio-Rad C1000 thermal cycler using gene-specific primers and iTaq Universal SYBR Green Supermix (Bio-Rad). Quantification of relative differences in gene expression was calculated using the 2−ΔΔCt methods, with Gapdh serving as a housekeeping reference.
Primer sequences were as follows:
| Gene | Forward, 5′-3′ | Reverse, 5′-3′ |
| ALP | ATGGGATGGGTGTCTCCACA | CCACGAAGGGGAACTTGTC |
| Collagen I | ACCGCCCTCCTGACGCAC | GCAGACGCAGATCCGGCAG |
| GAPDH | CAAGGCTGAGAACGGGAAGC | AGGGGGCAGAGATGATGACC |
The predesigned primers for RUNX2, STAT1, STAT3, IFNG, and IFNGR were obtained from Sigma.
Western Blot.
After 6 d of osteogenesis induction, total protein of DPSCs was extracted by 1× RIPA buffer (Cell Signaling) following the manufacturer’s instructions. The BCA protein assay (Pierce) was performed to determine the protein concentration, so that an equal amount of protein from each sample was applied for electrophoresis. Protein was separated on a 4 to 12% NuPAGE gel (Invitrogen) and transferred to nitrocellulose membranes by the iBlot blotting system (Invitrogen). After blocking with 5% (wt/vol) BSA for 1 h at room temperature, the membranes were incubated with diluted primary antibodies overnight in a cold room with gentle shaking. The membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Cell Signaling) for 1 h at room temperature. Subsequently, the bands were developed with Pico or Dura ECL substrate (Thermo) and detected by the Bio-Rad ChemiDoc Imaging System.
Cytosol and Nuclear Fractionation.
After 6 d of osteogenesis induction, DPSCs were harvested by trypsinization and the cell aggregates were removed by passing through a mesh. The single cells were collected by centrifugation (250 × g, 5 min) and washed with DPBS. The cells were resuspended in 0.4% (vol/vol) Triton X-100 in DPBS supplemented with protease inhibitors, and incubated on ice for 10 min. After centrifugation (12,000 × g, 5 min), the supernatant was transferred to a new tube and saved as the cytosol fraction. The remaining pellet was washed with DPBS and resuspended in RIPA buffer and vortexed vigorously. After removal of the insoluble part (16,000 × g, 10 min), the supernatant was saved as the nuclear fraction.
STAT1 Overexpression and KD.
A Lenti ORF clone of human STAT1 transcript variant alpha (OriGene) was obtained, and the lentivirus was produced by using a Lenti-vpak Packaging Kit (OriGene) following the manufacturer’s instructions. MISSION pLKO.1-puro Non-Mammalian shRNA Control Plasmid DNA (SHC002) and MISSION shRNA bacterial clone (SHCLNG) TRCN0000004265 for KD of STAT1 were purchased from Sigma. The lentivirus was packaged by using MISSION Lentiviral Packaging Mix (Sigma).
Flow Cytometry.
DPSCs were harvested by trypsinization and resuspended in serum-free DPSC growth medium. Cells were aliquoted into 1.5-mL tubes, and incubated with or without IFNG for the designated time at 37 °C with gentle shaking. Then, cells were chilled on ice for 5 min, collected by centrifugation, and transferred to a v-bottom 96-well plate on ice. Isotype control or CD130 (gp130)-PE (BD Biosciences) was added and incubated for 1 h. After removal of labeling antibodies, cells were resuspended in ice-cold FACS buffer and analyzed by an LSR II flow cytometer (Becton Dickinson).
Scanning Electron Microscopy.
The cells were fixed in 2.5% (vol/vol) glutaraldehyde in 0.1 M cacodylate buffer, and a small volume was placed on a 13-mm Whatman PC filter. The cells were rinsed on the filter with PBS. The entire double filter then was clamped between nylon washers, and the unit was placed in 1% (wt/vol) osmium tetroxide. After a PBS and water wash, the entire unit was dehydrated in ethanol and placed in a critical point dryer (Autosamdri 815, Tousimis). The nylon washers then were dismantled, the filters were separated, and both filters were mounted onto scanning electron microscope stubs with carbon tape. The stubs with attached filters then were sputter-coated with iridium for subsequent examination and documentation on a Hitachi S-4800 scanning electron microscope.
Human Lung Fibroblasts.
Primary human lung fibroblasts were purchased from Lonza (CC-2512) and maintained in FGM-2 Fibroblast Growth Medium-2 (CC-3132; Lonza). normal human lung fibroblasts from passages 2 and 3 were used in the studies.
Statistical Analysis.
Data are expressed as means ± SD. Statistical analysis was performed using the Student’s t test or by one-way ANOVA and the post hoc test. P values < 0.05 were considered significant.
Acknowledgments
This work was supported by the Jeffry M. and Barbara Picower (JPB) Foundation and Zebra Biologics.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706915114/-/DCSupplemental.
References
- 1.Germain RN. Maintaining system homeostasis: The third law of Newtonian immunology. Nat Immunol. 2012;13:902–906. doi: 10.1038/ni.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong Y, Sun Q, Zhang X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget. 2017;8:2171–2186. doi: 10.18632/oncotarget.13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bezbradica JS, Medzhitov R. Integration of cytokine and heterologous receptor signaling pathways. Nat Immunol. 2009;10:333–339. doi: 10.1038/ni.1713. [DOI] [PubMed] [Google Scholar]
- 5.Xie J, Zhang H, Yea K, Lerner RA. Autocrine signaling based selection of combinatorial antibodies that transdifferentiate human stem cells. Proc Natl Acad Sci USA. 2013;110:8099–8104. doi: 10.1073/pnas.1306263110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yea K, et al. Converting stem cells to dendritic cells by agonist antibodies from unbiased morphogenic selections. Proc Natl Acad Sci USA. 2013;110:14966–14971. doi: 10.1073/pnas.1313671110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxter GT, Kuo RC, Jupp OJ, Vandenabeele P, MacEwan DJ. Tumor necrosis factor-alpha mediates both apoptotic cell death and cell proliferation in a human hematopoietic cell line dependent on mitotic activity and receptor subtype expression. J Biol Chem. 1999;274:9539–9547. doi: 10.1074/jbc.274.14.9539. [DOI] [PubMed] [Google Scholar]
- 8.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 9.Dunne A, O’Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: Signal transduction during inflammation and host defense. Sci STKE. 2003;2003:re3. doi: 10.1126/stke.2003.171.re3. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto A, Sakai K, Matsubara K, Kano F, Ueda M. Multifaceted neuro-regenerative activities of human dental pulp stem cells for functional recovery after spinal cord injury. Neurosci Res. 2014;78:16–20. doi: 10.1016/j.neures.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Gandia C, et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2008;26:638–645. doi: 10.1634/stemcells.2007-0484. [DOI] [PubMed] [Google Scholar]
- 12.Akkouch A, Zhang Z, Rouabhia M. Engineering bone tissue using human dental pulp stem cells and an osteogenic collagen-hydroxyapatite-poly (L-lactide-co-ε-caprolactone) scaffold. J Biomater Appl. 2014;28:922–936. doi: 10.1177/0885328213486705. [DOI] [PubMed] [Google Scholar]
- 13.Iohara K, et al. Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng Part A. 2011;17:1911–1920. doi: 10.1089/ten.TEA.2010.0615. [DOI] [PubMed] [Google Scholar]
- 14.Wozney JM, et al. Novel regulators of bone formation: Molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 15.Bravo J, Heath JK. Receptor recognition by gp130 cytokines. EMBO J. 2000;19:2399–2411. doi: 10.1093/emboj/19.11.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka M, et al. Reconstitution of the functional mouse oncostatin M (OSM) receptor: Molecular cloning of the mouse OSM receptor beta subunit. Blood. 1999;93:804–815. [PubMed] [Google Scholar]
- 17.Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annu Rev Cell Dev Biol. 2009;25:629–648. doi: 10.1146/annurev.cellbio.042308.113308. [DOI] [PubMed] [Google Scholar]
- 18.Brodsky B, Baum J. Structural biology: Modelling collagen diseases. Nature. 2008;453:998–999. doi: 10.1038/453998a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merry K, Dodds R, Littlewood A, Gowen M. Expression of osteopontin mRNA by osteoclasts and osteoblasts in modelling adult human bone. J Cell Sci. 1993;104:1013–1020. doi: 10.1242/jcs.104.4.1013. [DOI] [PubMed] [Google Scholar]
- 20.Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357:1777–1789. doi: 10.1016/S0140-6736(00)04904-7. [DOI] [PubMed] [Google Scholar]
- 21.Calò V, et al. STAT proteins: From normal control of cellular events to tumorigenesis. J Cell Physiol. 2003;197:157–168. doi: 10.1002/jcp.10364. [DOI] [PubMed] [Google Scholar]
- 22.Gil MP, et al. Biologic consequences of Stat1-independent IFN signaling. Proc Natl Acad Sci USA. 2001;98:6680–6685. doi: 10.1073/pnas.111163898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S, et al. Stat1 functions as a cytoplasmic attenuator of Runx2 in the transcriptional program of osteoblast differentiation. Genes Dev. 2003;17:1979–1991. doi: 10.1101/gad.1119303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: Implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auguste P, et al. Signaling of type II oncostatin M receptor. J Biol Chem. 1997;272:15760–15764. doi: 10.1074/jbc.272.25.15760. [DOI] [PubMed] [Google Scholar]
- 26.Hibi M, et al. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63:1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Fuller GM. Phosphorylation and internalization of gp130 occur after IL-6 activation of Jak2 kinase in hepatocytes. Mol Biol Cell. 1994;5:819–828. doi: 10.1091/mbc.5.7.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honke N, et al. The p38-mediated rapid down-regulation of cell surface gp130 expression impairs interleukin-6 signaling in the synovial fluid of juvenile idiopathic arthritis patients. Arthritis Rheumatol. 2014;66:470–478. doi: 10.1002/art.38245. [DOI] [PubMed] [Google Scholar]
- 29.Gibson RM, et al. Phosphorylation of human gp130 at Ser-782 adjacent to the di-leucine internalization motif. Effects on expression and signaling. J Biol Chem. 2000;275:22574–22582. doi: 10.1074/jbc.M907658199. [DOI] [PubMed] [Google Scholar]
- 30.Scaffidi AK, et al. Oncostatin M stimulates proliferation, induces collagen production and inhibits apoptosis of human lung fibroblasts. Br J Pharmacol. 2002;136:793–801. doi: 10.1038/sj.bjp.0704769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canady J, Arndt S, Karrer S, Bosserhoff AK. Increased KGF expression promotes fibroblast activation in a double paracrine manner resulting in cutaneous fibrosis. J Invest Dermatol. 2013;133:647–657. doi: 10.1038/jid.2012.389. [DOI] [PubMed] [Google Scholar]
- 32.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferro F, et al. Dental pulp stem cells differentiation reveals new insights in Oct4A dynamics. PLoS One. 2012;7:e41774. doi: 10.1371/journal.pone.0041774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Y, et al. Effects of leukemia inhibitory factor on proliferation and odontoblastic differentiation of human dental pulp cells. J Endod. 2011;37:819–824. doi: 10.1016/j.joen.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 35.Cheon H, Stark GR. Unphosphorylated STAT1 prolongs the expression of interferon-induced immune regulatory genes. Proc Natl Acad Sci USA. 2009;106:9373–9378. doi: 10.1073/pnas.0903487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer T, Begitt A, Lödige I, van Rossum M, Vinkemeier U. Constitutive and IFN-gamma-induced nuclear import of STAT1 proceed through independent pathways. EMBO J. 2002;21:344–354. doi: 10.1093/emboj/21.3.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer T, Gavenis K, Vinkemeier U. Cell type-specific and tyrosine phosphorylation-independent nuclear presence of STAT1 and STAT3. Exp Cell Res. 2002;272:45–55. doi: 10.1006/excr.2001.5405. [DOI] [PubMed] [Google Scholar]
- 38.Yang J, Stark GR. Roles of unphosphorylated STATs in signaling. Cell Res. 2008;18:443–451. doi: 10.1038/cr.2008.41. [DOI] [PubMed] [Google Scholar]
- 39.Duque G, et al. Autocrine regulation of interferon gamma in mesenchymal stem cells plays a role in early osteoblastogenesis. Stem Cells. 2009;27:550–558. doi: 10.1634/stemcells.2008-0886. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, et al. Mesenchymal stem cell-based tissue regeneration is governed by recipient T lymphocytes via IFN-γ and TNF-α. Nat Med. 2011;17:1594–1601. doi: 10.1038/nm.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]