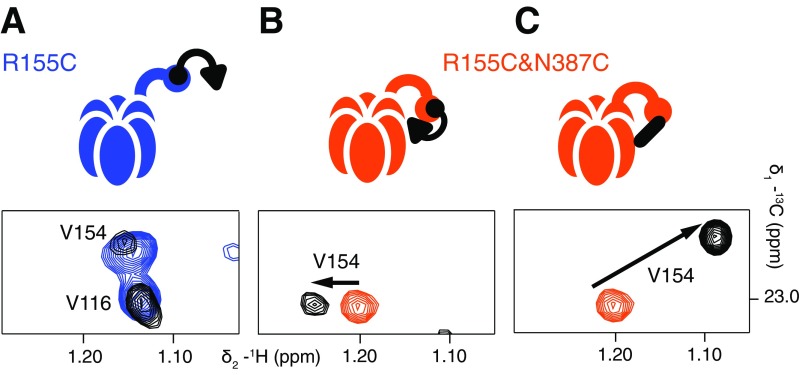
Fig. S4.
V154 is a proxy for disulfide bond formation between proximal C155 and C387 residues in R155C,N387C ND1Lp97-ADP. Selected regions of 13C-1H HMQC spectra are shown highlighting the resonance position of the δ1 methyl group of V154. (A) R155C ND1Lp97-ADP (blue, no UBXD1-N; black, threefold excess UBXD1-N over p97 protomers). As indicated schematically above the spectrum, binding of UBXD1-N to the NTD occurs via the VIM domain (black circle) but the H1/H2 motif (black triangle) is not able to interact, and there is no change in the peak position of V154. (B) In the R155C,N387C ND1Lp97-ADP variant, the up/down equilibrium is restored to the WT situation and WT two-pronged binding of UBXD1-N is observed (see main text and schematic). The position of V154 shifts slightly to reflect this, as indicated by the arrow. Sample is prepared with 5 mM TCEP. (C) A large chemical shift change is observed for V154 δ1 that reflects formation of the 155–387 disulfide in R155C,N387C ND1Lp97-ADP under oxidizing conditions (orange, 5 mM TCEP; black, oxidizing conditions). UBXD1-N is not added in this case.