Abstract
Objective: To evaluate the potential adjuvant effect of Agrocybe aegerita lectin (AAL), which was isolated from mushroom, against a virulent H9N2 strain in vivo and in vitro. Methods: In trial 1, 50 BALB/c male mice (8 weeks old) were divided into five groups (n=10 each group) which received a subcutaneous injection of inactivated H9N2 (control), inactivated H9N2+0.2% (w/w) alum, inactivated H9N2+0.5 mg recombinant AAL/kg body weight (BW), inactivated H9N2+1.0 mg AAL/kg BW, and inactivated H9N2+2.5 mg AAL/kg BW, respectively, four times at 7-d intervals. In trial 2, 30 BALB/c male mice (8 weeks old) were divided into three groups (n=10 each group) which received a subcutaneous injection of inactivated H9N2 (control), inactivated H9N2+2.5 mg recombinant wild-type AAL (AAL-wt)/kg BW, and inactivated H9N2+2.5 mg carbohydrate recognition domain (CRD) mutant AAL (AAL-mutR63H)/kg BW, respectively, four times at 7-d intervals. Seven days after the final immunization, serum samples were collected from each group for analysis. Hemagglutination assay, immunogold electron microscope, lectin blotting, and co-immunoprecipitation were used to study the interaction between AAL and H9N2 in vitro. Results: IgG, IgG1, and IgG2a antibody levels were significantly increased in the sera of mice co-immunized with inactivated H9N2 and AAL when compared to mice immunized with inactivated H9N2 alone. No significant increase of the IgG antibody level was detected in the sera of the mice co-immunized with inactivated H9N2 and AAL-mutR63H. Moreover, AAL-wt, but not mutant AAL-mutR63H, adhered to the surface of H9N2 virus. The interaction between AAL and the H9N2 virus was further demonstrated to be associated with the CRD of AAL binding to the surface glycosylated proteins, hemagglutinin and neuraminidase. Conclusions: Our findings indicated that AAL could be a safe and effective adjuvant capable of boosting humoral immunity against H9N2 viruses in mice through its interaction with the viral surface glycosylated proteins, hemagglutinin and neuraminidase.
Keywords: Adjuvant, Agrocybe aegerita lectin, Carbohydrate recognition domain, Glycosylated protein, Avian influenza H9N2 virus
1. Introduction
In many countries, avian influenza H9N2 virus is still a major threat to the poultry industry. It causes a huge economic burden when it leads to mild respiratory abnormality, reduced egg production, immune suppressive disorders, and even mortality (Li et al., 2011). Development of effective and safe vaccines is a priority in preparation for an influenza pandemic. Although vaccine has proved highly immunogenic in laboratory trials and prevents clinical diseases and reduces viral shedding in field conditions (Choi et al., 2008), it cannot prevent vaccinated poultry from becoming infected and from shedding wild viruses in farm settings (Rahman et al., 2012; Dabaghian et al., 2014). Hence, it is important to improve vaccination strategies to control H9N2 outbreaks in poultry farms.
The immune-promoting activity of any given vaccination strategy is determined not only by the presence of the relevant antigenic components in the vaccine formulation, but also by the addition of suitable adjuvants which activate, promote, and extend the magnitude of a specific immune response (Liu, 1998; Li et al., 2011). Emphasis is therefore being placed on the development of effective adjuvants to improve the efficiency of vaccines, especially inactivated viral and subunit vaccines.
Many substances have been used as adjuvants in experimental vaccines, such as aluminum, Freund’s complete adjuvant (FCA), immune stimulating complexes (ISCOMs), liposomes, polymeric microparticles, and saponins (Rajput et al., 2007). Most of them have major disadvantages, including toxicity, allergy, poor activity, and potential neurotoxicity, which limit their practical application (Sivakumar et al., 2011). Since numerous Chinese herbal medicines have been shown to have immune-enhancing effects (Yang et al., 2008), and also have many other advantages such as extensive availability, low cost, reliable efficacy, and decreased risk of side-effects and toxicity (Kong et al., 2004; 2006; Ung et al., 2007), their potential use as new adjuvants for humans or animals has aroused much research interest in recent years.
Agrocybe aegerita lectin (AAL) is a 15.8-kD homodimeric protein, isolated from the medicinal mushroom A. aegerita by our laboratory, which shows anti-tumor activity by inducing apoptosis in cancer cells (Zhao et al., 2003). We demonstrated that AAL belongs to a galectin family with a unique carbohydrate recognition domain (CRD) that specifically recognizes β-galactose (Yang et al., 2005; Feng et al., 2010). AAL has antifungal, antibacterial, antiviral (Sun et al., 2003), and antitumor functions and can improve immune function, and it is recognized that these functions are associated with the ability of its CRD to bind to glycan on glycosylated proteins (Rabinovich and Croci, 2012). We therefore hypothesized that AAL has adjuvant activity against the H9N2 virus by interacting with the glycosylated proteins, hemagglutinin (HA) and neuraminidase (NA) on the surface of the virus (Liu et al., 2003).
2. Materials and methods
2.1. Vaccine preparation of AAL, AAL-wt, and AAL-mutR63H
Avian influenza H9N2 virus (Academy of Veterinary Medicine, Huazhong Agricultural University, Wuhan, China) was cultured in 9-d-old embryonic eggs at 37 °C for 48 h. The allantoic fluid containing the viruses was harvested and purified by sucrose density gradient centrifugation. Purified vaccine viruses were inactivated by incubating at 75 °C for 15 min. The AAL from A. aegerita was extracted and purified by the chromatography method as previously described (Zhao et al., 2003). Arginine in the 63th locus of the CRD region of AAL (Arg63) is involved in the determination of the binding affinity of AAL to antigens (Feng et al., 2010). Wild-type AAL (AAL-wt) and CRD mutant AAL (AAL-mutR63H in which histidine replaced arginine in the 63th locus of the CRD region) with a C-terminal 6× His-tag were expressed in Escherichia coli BL21 (DE3) and purified as previously described (Liang et al., 2009). The recombinant AALs (with His-tag) were confirmed by anti-His antibody (Bioss, Beijing, China).
2.2. Mice, treatments, and sample collection
The Institutional Animal Care and Use Committee of Huazhong Agricultural University, China approved our animal protocol. In experiment 1, 50 BALB/c male mice (8 weeks old; Experimental Animal Center, Wuhan University, Wuhan, China) were divided into five groups (n=10 each group). All mice were allowed free access to commercial rodent diets (Yamaguchi et al., 2005) and water. After a week of acclimatization, the control group received a subcutaneous injection of 0.1 ml inactivated H9N2, and the alum group received a subcutaneous injection of 0.1 ml inactivated H9N2 with adjuvants of 0.2% (w/w) alum (Thermo, USA). The three other treated groups received a subcutaneous injection of 0.1 ml inactivated H9N2 with 0.5, 1.0, or 2.5 mg AAL/kg body weight (BW), respectively, four times at 7 d intervals (Huang et al., 2008; Kim et al., 2011). The inactivated viruses were mixed with the recombinant protein just prior to injection. Seven days after the final immunization the sera were harvested from the immunized mice (Kim et al., 2011). The sera were prepared by centrifugation of the whole blood at 1000g for 15 min at 4 °C and stored at −80 °C (Sun et al., 2014). In experiment 2, 30 BALB/c male mice (8 weeks old) were divided into three groups (n=10 each group) and received the same housing conditions as in experiment 1. The control group received a subcutaneous injection of 0.1 ml inactivated H9N2, while the two treated groups received a subcutaneous injection of 0.1 ml inactivated H9N2 with adjuvants of 2.5 mg AAL-wt/kg BW and 2.5 mg AAL-mutR63H/kg BW, respectively, four times at 7 d intervals (Huang et al., 2008; Kim et al., 2011). Seven days after the final immunization, serum samples were collected following the method described by Kim et al. (2011). No negative effects on the experimental animals were observed.
2.3. Measurement of antigen-specific antibodies
The H9N2-specific IgG, IgG1, and IgG2a antibodies in the sera were analyzed by enzyme-linked immunosorbent assay (ELISA) as described previously with minor modifications (Sun and Liu, 2008). Briefly, the 96-well microtiter plates were coated with 100 μl of H9N2 solution (50 μg/ml in 50 mmol/L carbonate buffer, pH 9.6) for 24 h at 4 °C. The wells were washed three times with 200 μl of phosphate buffer saline (PBS) containing 0.05% (v/v) Tween 20 (PBST), and then blocked with 100 μl of PBST within 0.5% (v/v) bovine serum albumin (BSA) at 37 °C for 1 h. One hundred microliters of diluted serum samples were then added to triplicate wells, and then incubated at 37 °C for 1 h, followed by washing three times. Aliquots of 100 μl of horseradish peroxidase-conjugated goat anti-mouse IgG, IgG1, or IgG2a (SouthernBiotech, China) were added and incubated for 1 h at 37 °C. An assay was developed using 3,3',5,5'-tetramethylbenzidine (TMB) (Beytime Biotechnology, China), and the reaction was stopped by adding 0.5 mol/L H2SO4. The absorbance at 450 nm was measured by an ELISA plate reader.
2.4. Hemagglutinating assays
The hemagglutinating assays were performed as described previously with minor modifications (Yang et al., 2005). Briefly, a serial two-fold dilution of AAL-wt or AAL-mutR63H solution in microtiter U-plates (50 µl) was mixed with 50 µl of 1.0% suspension of mouse erythrocytes in PBS (pH 7.2) at 20 °C. The initial concentration was 1 mg/ml. The results were read after about 1 h, when the control (no AAL added) had fully precipitated. The hemagglutination titer, defined as the reciprocal of the highest dilution exhibiting hemagglutination, was defined as one hemagglutination unit. Specific activity was the number of hemagglutination units per mg of protein.
2.5. Immunogold electron microscope assays
Immunogold electron microscope assays were performed as described previously (Pena et al., 2013). Briefly, 10 μl purified H9N2 viruses were adsorbed onto formvar-coated and carbon-vaporized 300-mesh copper grids (Electron Microscopy Sciences, Hatfield, PA, USA). The grids were blocked in Tris-buffered saline (TBS) containing 0.2% (v/v) BSA at room temperature (RT) for 1 h, and washed three times with TBS with 0.3% Tween 20 (TBST). They were then incubated with TBS (control), 10 μl AAL-wt (500 ng/ml), or AAL-mutR63H (500 ng/ml) at RT for 1 h. Subsequently, the grids were washed in TBS and incubated with rabbit anti-His (anti-AAL) at RT for 1 h, and then incubated with goat anti-rabbit IgG antibody conjugated to 15-nm gold beads (Bioss, Beijing, China) at RT for 1 h. Finally, the grids were negatively stained with 2% (v/v) phosphotungstic acid (PTA) for 30 min, dried, and examined under a transmission electron microscope (Hitachi H-7000FA).
2.6. Lectin blot assays
Lectin blot assays were performed as described previously with minor modifications (Matsumura et al., 2007). Briefly, the protein extracts of the H9N2 virus were isolated by methanol and chloroform, and 60 μg of the extracted protein was then subjected to 15% (0.15 g/ml) sodium dodecyl sulfate-polyarylamide gel electrophoresis (SDS-PAGE). One of the samples was treated with PNGase F (New England BioLabs, MA, USA) to remove the glycan on glycosylated protein following the manufacturer’s instructions. After electrophoresis, the gels were blotted onto polyvinylidene difluoride (PVDF) membranes which were incubated overnight with 5% (v/v) BSA in TBST (50 mmol/L Tis-HCl, 150 nmol/L NaCl, 0.1% Tween 20, pH 7.4) at 4 °C, and then for 100 min with 500 ng/ml of AAL-wt or AAL-mutR63H in TBST. After being washed with TBST, the membranes were incubated with anti-His (anti-AAL) antibody (Bioss, Beijing, China) at RT for 100 min. Staining was then performed with ECL Western blot detection reagents as previously described (Sun et al., 2013).
2.7. Co-immunoprecipitation assays
Co-immunoprecipitation (Co-IP) assays were performed as described previously (Rajik et al., 2009). Briefly, the extracted protein of H9N2 virus was diluted to a concentration of 1–2 μg/μl in 50 μl Co-IP buffer, and was then incubated overnight with 50 μl of 100 μmol/L AAL-wt at 4 °C. Anti-His (anti-AAL) antibody, anti-HA antibody (Sino Biological Inc., Beijing, China), and IgG antibody were added to the mixture, respectively, and rotated gently for 3.5 h at 4 °C. Subsequently, protein G plus-agarose beads were added, and the mixture was rotated gently for 3 h at 4 °C. The beads were collected by centrifugation at 100g for 3 min at 4 °C, and then washed three times with ice-cold NP40 lysis buffer. Finally, the proteins were eluted from the beads by boiling in SDS loading buffer for 5 min, and analyzed by Western blot assay using the anti-His (anti-AAL) antibody and anti-HA antibody with appropriate secondary antibodies.
2.8. Statistical analysis
Data generated from animal studies were analyzed using Student’s t-test by using SAS 8.2 (SAS Institute). Data are presented as mean±standard error (SE) with the significance level set at P<0.05.
3. Results
3.1. Adjuvant activity of AAL on antibody titers against H9N2 in mice
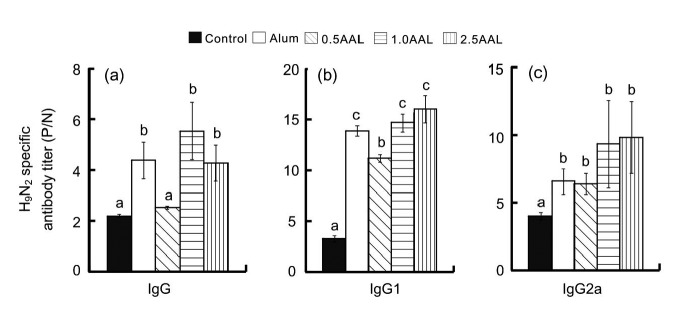
AAL was successfully extracted and purified by chromatography and confirmed by anti-AAL for Western blot assay (Figs. 1a and 1d). In the first animal experiment, after 4 weeks of treatment, compared to mice immunized with H9N2 alone (control), IgG antibody levels in the sera were increased (P<0.05) by 97%, 147%, and 93% when mice were co-immunized with inactivated H9N2 and alum, AAL (1.0 mg/kg BW), and AAL (2.5 mg/kg BW), respectively (Fig. 2a). IgG1 antibody levels in the sera were increased (P<0.05) by 237%, 340%, and 372% when mice were co-immunized with inactivated H9N2 and AAL at the doses of 0.5, 1.0, and 2.5 mg/kg BW, respectively (Fig. 2b). In addition, IgG2a antibody levels in the sera were increased (P<0.05) by 60%, 133%, and 145% when mice were co-immunized with inactivated H9N2 and AAL at the doses of 0.5, 1.0, and 2.5 mg/kg BW, respectively (Fig. 2c).
Fig. 1.
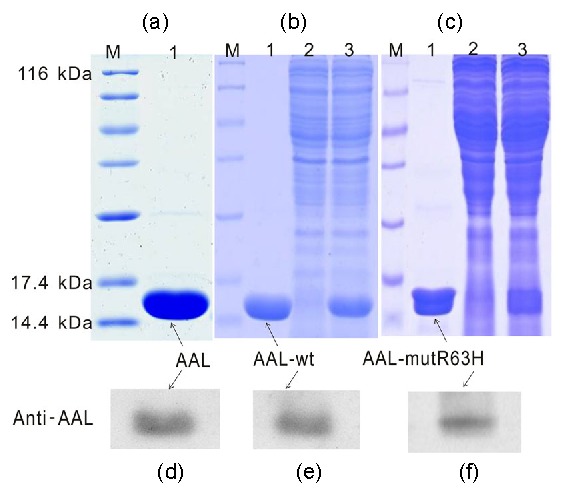
Purified AAL (a), AAL-wt (b), and AAL-mutR63H (c) applied to 15% SDS-PAGE and stained with Coomassie brilliant blue, and Western blot analysis of the purified AAL (d), AAL-wt (e), and AAL-mutR63H (f) using rabbit anti-AAL antibody
M, sizes (kD) of molecular weight markers; Lane 1, purified AAL, AAL-wt, or AAL-mutR63H; Land 2, flow-through; Land 3, crude extract AAL
Fig. 2.
Effects of co-immunization with inactivated H9N2 and AAL or alum on IgG (a), IgG1 (b), and IgG2a (c) antibody levels in sera of mice
Values are expressed as mean±SE, n=10 each group. Values without sharing a common letter differ significantly (P<0.05). 0.5AAL: 0.5 mg AAL/kg BW; 1.0AAL: 1.0 mg AAL/kg BW; 2.5AAL: 2.5 mg AAL/kg BW
3.2. Hemagglutinating and adjuvant activity assays of AAL-wt and AAL-mutR63H
To analyze the role of the CRD of AAL by site-directed mutagenesis, Arg63 at the domain was replaced with His63 based on the three-dimensional structures of AAL to produce the mutated AAL (AAL-mutR63H) which was then purified and confirmed by Western blot. This showed that Arg63 is a key function site of AAL (Figs. 1c and 1f) (Feng et al., 2010). We also produced and purified the recombinant AAL-wt and confirmed it by Western blot (Figs. 1b and 1e). The hemagglutinating assay showed that AAL-wt has hemagglutinating activity (1:2–1:128), while no activity was found in AAL-mutR63H (Fig. 3a). The second animal experiment showed that AAL-wt significantly enhanced (P<0.05) H9N2-specific IgG levels in the sera of mice, but that AAL-mutR63H had no significant effect (P>0.05) (Fig. 3b).
Fig. 3.
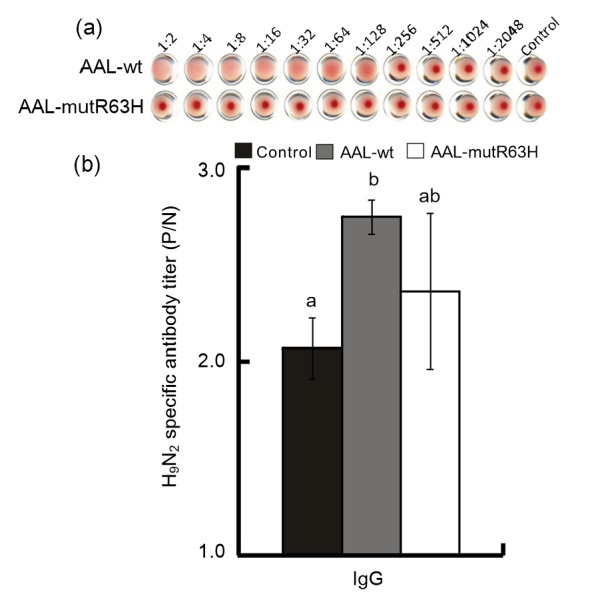
Effects of AAL-wt and AAL-mutR63H on hemagglutinating activity (a) and IgG antibody level (b) in the sera of mice
Values are expressed as mean±SE, n=10 each group. Values without sharing a common letter differ significantly (P<0.05)
3.3. Immunogold electron microscope, lectin blot, and co-immunoprecipitation assays
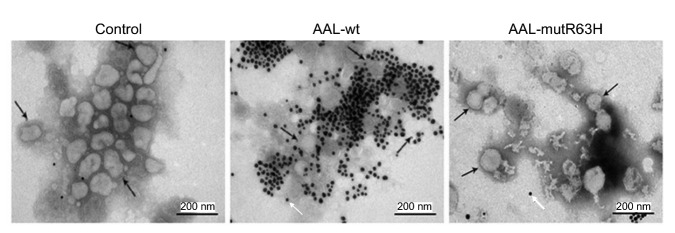
The immunogold electron microscope results showed that, compared with the control group, AAL-wt accumulated intensively on the surface of the H9N2 virus, but only a few of the mutant AAL-mutR63H did (Fig. 4). To test whether the two glycosylated proteins, HA and NA, on the surface of the H9N2 virus could be the binding targets of AAL’s CRD, we preformed lectin blot analysis (Fig. 5a). The results showed that both glycoproteins NA (55 kD) and HA (26 kD) were displayed on the AAL-wt-incubated PVDF membrane (Land 1); however, these two bands disappeared after the N-linked glycan of virus was removed by PNGase F (Land 2) or in the AAL-mutR63H-incubated PVDF membrane (Land 3). These data indicated that AAL interacted with the glycosylated proteins HA and NA, while the mutant AAL-mutR63H lost its ability to interact with these two proteins. The interaction of AAL with HA was further confirmed by Co-IP assay (Fig. 5b). Protein extracts of H9N2 virus were incubated with AAL-wt/AAL-mutR63H, and then with anti-HA, anti-AAL, and anti-IgG antibodies. Finally, the total extracts and the precipitates were Western-blotted against anti-HA and anti-AAL (Fig. 5b). HA/AAL-wt was detected in AAL-wt/HA immunoprecipitates with H9N2 virus protein extracts, indicating that the HA/AAL-wt was captured by the anti-AAL-wt/HA antibody. The Co-IP results further indicated that AAL interacted with the glycosylated proteins HA. In contrast, no interaction was found between HA and AAL-mutR63H.
Fig. 4.
Immunogold electron microscope analysis of the interaction between avian influenza H9N2 virus and the CRDs of AAL-wt and AAL-mutR63H
Black arrow: H9N2 virus; White arrow: gold particle
Fig. 5.
Lectin blot (a) and co-immunoprecipitation (Co-IP) (b) analysis of the interaction between avian influenza H9N2 viral surface glycosylated proteins, HA and NA, and the CRDs of AAL-wt and AAL-mutR63H
Input is mixed HA with AAL-wt or AAL-mutR63H
4. Discussion
The two novel findings from the present study are: (1) AAL has an adjuvant activity against avian influenza H9N2 virus in mice; (2) the mechanism of AAL’s adjuvant activity is associated with the interaction between the CRD of AAL with the H9N2 virus surface glycosylated proteins HA and NA. Levels of total IgG, IgG1, and IgG2a in serum have been described as valuable parameters of humoral immunity (Li et al., 2011). The first animal study showed that co-injecting inactivated H9N2 virus with ≥1.0 mg AAL/kg BW induced higher levels of antigen-specific IgG, IgG1, and IgG2a antibody titers in mice than injecting inactivated H9N2 avian influenza virus (AIV) vaccine alone. Furthermore, the levels of IgG, IgG1, and IgG2a were similar in the mice co-injected with inactivated H9N2 virus with AAL or alum. These outcomes indicated that AAL, like alum, has an effective adjuvant activity that enhances antigen-specific humoral immune responses. Similar results were obtained from recent studies in which Korean mistletoe lectin was found to have the ability to serve as a mucosal adjuvant (Jung et al., 2011) and mushroom lectin as an adjuvant of hepatitis B virus (HBV) DNA vaccine (Gao et al., 2013). AAL is attractive as a new adjuvant for animal H9N2 vaccines, since it can be isolated from mushroom A. aegerita, which is extensively available and has reliable efficacy but lower risks of side-effects and toxicity than alum salts or other adjuvants (Aguilar and Rodríguez, 2007; Sivakumar et al., 2011).
To validate our hypothesis that the CRD plays a vital role in AAL’s adjuvant activity, we obtained AAL-mutR63H, by replacing Arg63 at CRD with His63 (Feng et al., 2010), and studied its biological function. The in vitro study showed that AAL-wt had haemagglutination activity, but that AAL-mutR63H lost this activity. The second animal study showed that AAL-wt enhanced the levels of IgG in the sera of mice, while AAL-mutR63H had no significant effect. Taken together, these outcomes indicated that the CRD of AAL plays a key role in the adjuvant effect of AAL against the H9N2 virus in mice. Similar results were obtained by Dodson et al. (1999), who reported that the CRD of the Entamoeba histolytica Gal/GalNAc lectin played key roles in infection and immunity.
Since lectin’s antiviral function and ability to improve immune function are associated with the ability of its CRD to bind to glycan on glycosylated protein (Rabinovich and Croci, 2012), we carried out immunogold electron microscope, lectin blot, and Co-IP assays to determine whether AAL’s adjuvant activity against the H9N2 virus was associated with the ability of its CRD to bind to the surface glycosylated proteins HA and NA. The immunogold electron microscope results showed that AAL-wt bound strongly to the surface of the H9N2 virus, but the mutant AAL-mutR63H had lost this ability. Since HA and NA are the two major glycosylated proteins on the surface (Liu et al., 2003), lectin blot and Co-IP were done to determine AAL’s ability to bind to the surface through its CRD interacting with these two glycosylated proteins. The lectin blot results showed that AAL-wt interacted with both HA and NA, but AAL-mutR63H lost its ability to interact with these two proteins. The interaction between AAL and the HA protein was further confirmed by Co-IP assay. Overall, the immunogold electron microscope, lectin blot, and Co-IP assays demonstrated that the CRD of AAL plays a vital role in interacting with the glycosylated proteins HA and NA on the surface of the H9N2 virus.
The glycosylation of viral envelope proteins plays an important role in viral immune evasion by masking antigenic sites from immune recognition (Kreisman and Cobb, 2012). Previous reports have shown that N-linked glycosylation can limit the neutralizing antibody response to simian immunodeficiency virus (SIV) and shield the virus from immune recognition (Reitter et al., 1998), and that the glycosylations of HA and NA are significant for influenza virus to evade the host immune system (Klenk et al., 2001; Tsuchiya et al., 2002a; 2002b). Therefore, we speculated that the enhancement of antigenicity of the H9N2 virus by AAL may be related to the interaction of AAL with glycosylated proteins on the virus surface by exposing antigenic sites to immune recognition (Fig. 6a). Since AAL is a homodimeric protein with two CRDs (Zhao et al., 2003), which may link two viral particles, an antigen depot is formed (Fig. 6b). The antigen depot can provide a slow release of antigen, which can continue the stimulation of the immune system (Lindblad, 2004).
Fig. 6.

Schematic illustration of potential mechanisms of AAL serving as an adjuvant of avian influenza H9N2 vaccine
(a) The enhancement of antigenicity of the H9N2 virus by AAL may be related to the interaction of AAL with the glycosylated proteins on the virus surface, resulting in the exposure of the antigenic sites to immune recognition. (b) AAL as a homodimeric protein with two CRDs may link two viral particles and form an antigen depot. The antigen depot can provide a slow release of antigen, continuing the stimulation of the immune system
5. Conclusions
In summary, AAL purified from mushroom A. aegerita and AAL-wt both exhibited adjuvant effects against avian influenza H9N2 virus. The site-directed mutant AAL-mutR63H (Arg63 at the CRD of AAL replaced with His63) lost haemagglutination activity in vitro and adjuvant activity in vivo, indicating that the CRD of AAL plays a vital role in the adjuvant effect against the H9N2 virus in mice. Furthermore, the immunogold electron microscope, lectin blot, and Co-IP assays demonstrated that the CRD of AAL interacted with the glycosylated proteins HA and NA on the surface of the H9N2 virus, which resulted in the exposure of the antigenic sites to immune recognition and the formation of an antigen depot. This may explain why the antigenicity of the H9N2 virus was enhanced. These findings indicate that the use of AAL as a novel adjuvant offers a new prophylaxis strategy against enveloped H9N2 viruses.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 30771501 and 81102850), the National Basic Research Program (973) of China (No. 2011CB811302), the National Mega Project on Major Drug Development (No. 2009ZX09301-014-1), the Chinese 111 Project (No. B06018), and the Wuhan Municipal Project (No. 201160923296), China
Compliance with ethics guidelines: Li-bao MA, Bao-yang XU, Min HUANG, Lv-hui SUN, Qing YANG, Yi-jie CHEN, Ya-lin YIN, Qi-gai HE, and Hui SUN declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Aguilar JC, Rodríguez EG. Vaccine adjuvants revisited. Vaccine. 2007;25(19):3752–3762. doi: 10.1016/j.vaccine.2007.01.111. [DOI] [PubMed] [Google Scholar]
- 2.Choi JG, Lee YJ, Kim YJ, et al. An inactivated vaccine to control the current H9N2 low pathogenic avian influenza in Korea. J Vet Med Sci. 2008;9(9):67–74. doi: 10.4142/jvs.2008.9.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dabaghian M, Latify AM, Tebianian M, et al. Vaccination with recombinant 4×M2e.HSP70c fusion protein as a universal vaccine candidate enhances both humoral and cell-mediated immune responses and decreases viral shedding against experimental challenge of H9N2 influenza in chickens. Vet Microbiol. 2014;174(1-2):116–126. doi: 10.1016/j.vetmic.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Dodson JM, Lenkowski PW, Eubanks AC, et al. Infection and immunity mediated by the carbohydrate recognition domain of the Entamoeba histolytica Gal/GalNAc lectin. J Infect Dis. 1999;179(2):460–466. doi: 10.1086/314610. [DOI] [PubMed] [Google Scholar]
- 5.Feng L, Sun H, Zhang Y, et al. Structural insights into the recognition mechanism between an antitumor galectin AAL and the Thomsen-Friedenreich antigen. FASEB J. 2010;24(10):3861–3868. doi: 10.1096/fj.10-159111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao W, Sun Y, Chen S, et al. Mushroom lectin enhanced immunogenicity of HBV DNA vaccine in C57BL/6 and HBsAg-transgenic mice. Vaccine. 2013;31(18):2273–2280. doi: 10.1016/j.vaccine.2013.02.062. [DOI] [PubMed] [Google Scholar]
- 7.Huang L, Ikejiri A, Shimizu Y, et al. Immunoadjuvant activity of crude lectin extracted from Momordica charantia seed. J Vet Med Sci. 2008;70(5):533–535. doi: 10.1292/jvms.70.533. [DOI] [PubMed] [Google Scholar]
- 8.Jung HJ, Kim YH, Song TJ, et al. Adjuvant effect of Korean mistletoe lectin on mucosal immunity induction following intranasal immunization with hemagglutinin antigen. Food Sci Biotechnol. 2011;20(3):629–634. doi: 10.1007/s10068-011-0089-3. [DOI] [Google Scholar]
- 9.Kim JC, Yoon TJ, Song TJ, et al. Mucosal immunoadjuvant activity of Korean mistletoe lectin-C. Korean J Food Sci Technol. 2011;43(1):72–76. doi: 10.9721/KJFST.2011.43.1.072. [DOI] [Google Scholar]
- 10.Klenk HD, Wagner R, Heuer D, et al. Importance of hemagglutinin glycosylation for the biological functions of influenza virus. Virus Res. 2001;82(1-2):73–75. doi: 10.1016/S0168-1702(01)00389-6. [DOI] [PubMed] [Google Scholar]
- 11.Kong X, Rui R, Li X, et al. Effects of Chinese herbal medicinal ingredients on peripheral lymphocyte proliferation and serum antibody titer after vaccination in chicken. Int Immunopharmacol. 2004;4(7):975–982. doi: 10.1016/j.intimp.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Kong XF, Hu YL, Yin YL, et al. Chinese herbal ingredients are effective immune stimulators for chickens infected with the Newcastle disease virus. Poultry Sci. 2006;85(12):2169–2175. doi: 10.1093/ps/85.12.2169. [DOI] [PubMed] [Google Scholar]
- 13.Kreisman LS, Cobb BA. Infection, inflammation and host carbohydrates: a Glyco-Evasion Hypothesis. Glycobiology. 2012;22(8):1019–1030. doi: 10.1093/glycob/cws070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li D, Xue M, Wang C, et al. Bursopentine as a novel immunoadjuvant enhances both humoral and cell-mediated immune responses to inactivated H9N2 avian influenza virus in chickens. Clin Vacc Immunol. 2011;18(9):1497–1502. doi: 10.1128/CVI.05133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004;82(5):497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 16.Liu JH, Okazaki K, Shi WM, et al. Phylogenetic analysis of hemagglutinin and neuraminidase genes of H9N2 viruses isolated from migratory ducks. Virus Genes. 2003;27(3):291–296. doi: 10.1023/A:1026304117797. [DOI] [PubMed] [Google Scholar]
- 17.Liu MA. Vaccine developments. Nat Med. 1998;4(5):515–519. doi: 10.1038/nm0598supp-515. [DOI] [PubMed] [Google Scholar]
- 18.Matsumura K, Higashida K, Ishida H, et al. Carbohydrate binding specificity of a fucose-specific lectin from Aspergillus oryzae: a novel probe for core fucose. J Biol Chem. 2007;282(21):15700–15708. doi: 10.1074/jbc.M701195200. [DOI] [PubMed] [Google Scholar]
- 19.Pena L, Sutton T, Chockalingam A, et al. Influenza viruses with rearranged genomes as live-attenuated vaccines. J Virol. 2013;87(9):5118–5127. doi: 10.1128/JVI.02490-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabinovich G, Croci D. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity. 2012;36(3):322–335. doi: 10.1016/j.immuni.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Rahman MM, Uyangaa E, Han YW, et al. Enhancement of Th1-biased protective immunity against avian influenza H9N2 virus via oral co-administration of attenuated Salmonella enterica serovar Typhimurium expressing chicken interferon-α and interleukin-18 along with an inactivated vaccine. BMC Vet Res. 2012;8:105. doi: 10.1186/1746-6148-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajik M, Jahanshiri F, Omar AR, et al. Identification and characterisation of a novel anti-viral peptide against avian influenza virus H9N2 . Virol J. 2009;6:74. doi: 10.1186/1743-422X-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajput ZI, Hu SH, Xiao CW, et al. Adjuvant effects of saponins on animal immune responses. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2007;8(3):153–161. doi: 10.1631/jzus.2007.B0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reitter JN, Means RE, Desrosiers RC. A role for carbohydrates in immune evasion in AIDS. Nat Med. 1998;4(6):679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 25.Sivakumar SM, Safhi MM, Kannadasan M, et al. Vaccine adjuvants–current status and prospects on controlled release adjuvancity. Saudi Pharm J. 2011;19(4):197–206. doi: 10.1016/j.jsps.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun H, Zhao CG, Tong X, et al. A lectin with mycelia differentiation and antiphytovirus activities from the edible mushroom Agrocybe aegerita . J Biochem Mol Biol. 2003;36(2):214–222. doi: 10.5483/BMBRep.2003.36.2.214. [DOI] [PubMed] [Google Scholar]
- 27.Sun LH, Li JG, Zhao H, et al. Porcine serum can be biofortified with selenium to inhibit proliferation of three types of human cancer cells. J Nutr. 2013;143(7):1115–1122. doi: 10.3945/jn.113.177410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun LH, Lei MY, Zhang NY, et al. Hepatotoxic effects of mycotoxin combinations in mice. Food Chem Toxicol. 2014;74:289–293. doi: 10.1016/j.fct.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Liu J. Adjuvant effect of water-soluble polysaccharide (PAP) from the mycelium of Polyporus albicans on the immune responses to ovalbumin in mice. Vaccine. 2008;26(31):3932–3936. doi: 10.1016/j.vaccine.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Tsuchiya E, Sugawara K, Hongo S, et al. Effect of addition of new oligosaccharide chains to the globular head of influenza A/H2N2 virus haemagglutinin on the intracellular transport and biological activities of the molecule. J Gen Virol. 2002;83(5):1137–1146. doi: 10.1099/0022-1317-83-5-1137. [DOI] [PubMed] [Google Scholar]
- 31.Tsuchiya E, Sugawara K, Hongo S, et al. Role of overlapping glycosylation sequons in antigenic properties, intracellular transport and biological activities of influenza A/H2N2 virus haemagglutinin. J Gen Virol. 2002;83(12):3067–3074. doi: 10.1099/0022-1317-83-12-3067. [DOI] [PubMed] [Google Scholar]
- 32.Ung CY, Li H, Kong CY, et al. Usefulness of traditionally defined herbal properties for distinguishing prescriptions of traditional Chinese medicine from non-prescription recipes. J Ethnopharmacol. 2007;109(1):21–28. doi: 10.1016/j.jep.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguchi N, Sonoyama K, Kikuchi H, et al. Gastric colonization of Candida albicans differs in mice fed commercial and purified diets. J Nutr. 2005;135(1):109–115. doi: 10.1093/jn/135.1.109. [DOI] [PubMed] [Google Scholar]
- 34.Yang L, Hu Y, Xue J, et al. Compound Chinese herbal medicinal ingredients can enhance immune response and efficacy of RHD vaccine in rabbit. Vaccine. 2008;26(35):4451–4455. doi: 10.1016/j.vaccine.2008.06.075. [DOI] [PubMed] [Google Scholar]
- 35.Yang N, Tong X, Xiang Y, et al. Molecular character of the recombinant antitumor lectin from the edible mushroom Agrocybe aegerita . J Biochem. 2005;138(2):145–150. doi: 10.1093/jb/mvi109. [DOI] [PubMed] [Google Scholar]
- 36.Liang Y, Lei F, Xin T, et al. Importance of nuclear localization for the apoptosis-induced activity of a fungal galectin AAL (Agrocybe aegerita lectin) Biochem Biophys Res Commun. 2009;386(3):437–442. doi: 10.1016/j.bbrc.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 37.Zhao C, Sun H, Tong X, et al. An antitumour lectin from the edible mushroom Agrocybe aegerita . Biochem J. 2003;374(2):321–327. doi: 10.1042/bj20030300. [DOI] [PMC free article] [PubMed] [Google Scholar]