1. Background
Cardiac arrhythmias are a frequent complication related to seizures [1]. Most are benign although some, such as high-grade atrioventricular block, asystole, atrial fibrillation (AF), and ventricular fibrillation are potentially harmful. Indeed, several studies suggest that cardiac dysfunction may be the primary underlying cause of sudden unexpected death in epilepsy (SUDEP). Post-ictal atrial fibrillation (PIAF) is a very rare cardiac arrhythmia associated with seizures, and its relevance is currently unknown. AF characteristics include: 1) irregular R-R intervals, 2) absence of distinct repeating P waves, and 3) erratic atrial activity [2]. Atrial flutter is differentiated from AF because of the characteristic “saw tooth” waves on the ECG instead of the chaotic atrial rhythm of the AF. In fact, there are only four reported cases of PIAF diagnosed during video-EEG monitoring (VEM) [1], [3], [4]. We report a case of PIAF identified during long-term VEM, with recurrent episodes corroborated by Holter-ECG monitoring.
2. Case report
A 32-year-old, right-handed male suffering from genetic drug-resistant epilepsy, with generalized tonic–clonic seizures (GTCS) from the age of 7, underwent VEM for differential diagnosis and syndromic classification. He presented severe language developmental delay and autistic behavior resulting in marked cognitive impairment, with no obvious phenotypic abnormality. No cardiovascular events or other medical conditions were recorded. A genomic hybridization analysis (chromosomal microarray) showed a 22q13 deletion (22q13.33) with deletion of SHANK3 gene corresponding to a Phelan–McDermid syndrome. A 3T brain MRI was normal. Previous EEG showed interictal multifocal abnormalities. A baseline 12-lead ECG was normal. The patient was treated with combination therapy including valproate, zonisamide, lacosamide, and perampanel after multiple antiepileptic drug trials. Interestingly, within the family history it was found that both maternal grandparents required the implantation of a pacemaker and that a maternal uncle also had an implantable cardioverter-defibrillator (ICD).
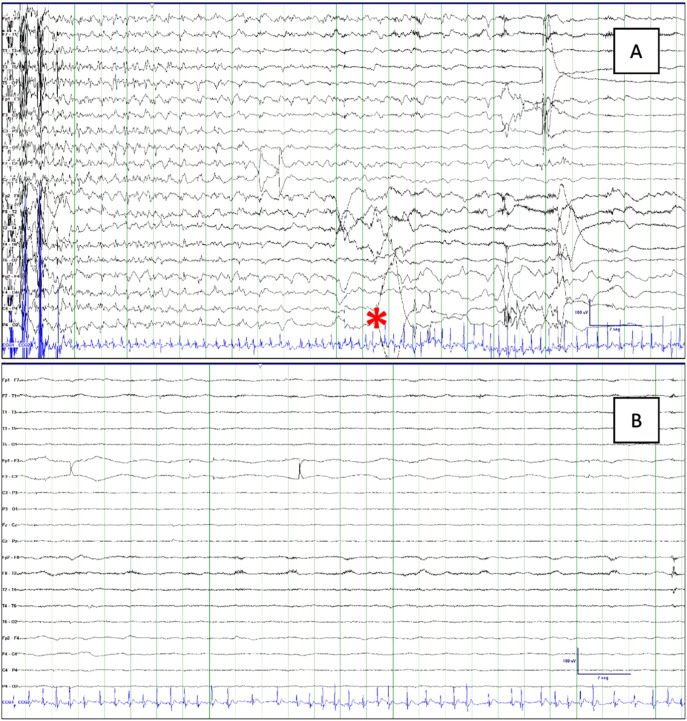
During admission, perampanel and lacosamide were withdrawn. The EEG showed a slow background rhythm (6–7 Hz). Interictal activity consisted of bifrontal symmetrical spike-and-waves and frontotemporal polyspikes. The patient experienced a GTCS that commenced with a bifrontal symmetrical spike–wave followed by bilateral symmetrical ictal activity. After the seizure he was unresponsive for 20 min, in association with a post-ictal generalized electroencephalographic suppression (PGES) pattern (Fig. 1).
Fig. 1.
Post-ictal EEG and ECG. (A) Seizure termination and beginning of atrial fibrillation (asterisk). (B) PGES during AF beginning immediately after seizure termination. EEG amplitude was < 10 μV and lasted 90 s.
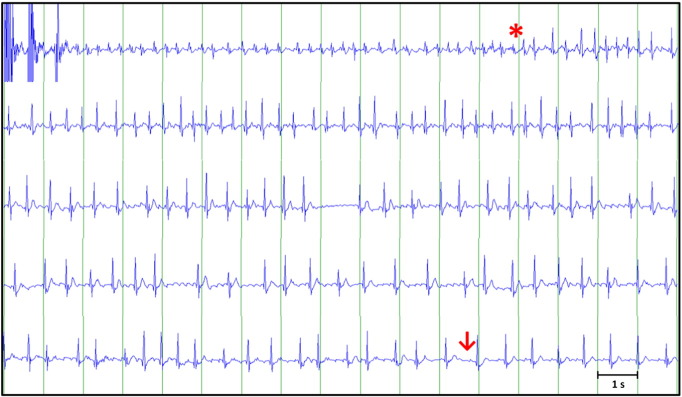
The ECG-channel of the video-EEG (XLTEK® EMU128FS) showed no abnormality during the 24 hours previous to the seizure. Muscle artifact precluded its visualization during the tonic–clonic phase, but when this ended a sinus tachycardia was observed. After 11 s, sinus rhythm converted into AF (Fig. 1, Fig. 2) that subsequently switched to atrial flutter (Fig. 2), lasting 4 min 40 s, and then reverted spontaneously to sinus rhythm (Fig. 2). After this episode, medication was reintroduced, with the exception of lacosamide, due to its potential arrhythmogenic properties, and no further seizures were recorded during VEM. An outpatient 21-day cardiac event ECG monitor showed a new episode of PIAF after a GTCS (marked by the mother) that started 5 s after the end of movement artifact, assumed to be the seizure end, lasting for 3 min. Later echocardiography showed no structural heart disease. The implantation of an implantable cardiac monitor for continuous monitoring and early detection of potentially malignant arrhythmias was performed.
Fig. 2.
Sample of post-ictal atrial fibrillation evolution. Post-ictal sinus tachycardia (first line), beginning of atrial fibrillation (asterisk), and posterior conversion into atrial flutter (third line). Return to sinus rhythm in the last line (arrow).
3. Discussion
According to the literature, PIAF is a very infrequent cardiac arrhythmia associated with seizures. There are only four reported cases of PIAF identified during VEM [1], [3], [4]. In these four cases, as well as ours, the duration of the arrhythmia was very short and therefore almost impossible to detect on an outpatient basis. It is thus probable that PIAF occurs more frequently than reported and is underdiagnosed.
Although the pathophysiological mechanisms of PIAF are not well defined, direct or indirect autonomic changes are likely to be involved. All but one reported case of PIAF occurred after a GTCS [1]. Following a GTCS there is a period of severe autonomic dysregulation dominated by excessive sympathetic activation coupled with parasympathetic suppression in the early postictal phase; whereas the later phase seems to be dominated by impaired vagal recovery. The magnitude and duration of these autonomic disturbances strongly correlate with PGES, which is associated with greater respiratory dysfunction, arousal impairment, and autonomic dysregulation. It is noteworthy in our case, since PGES began simultaneously with the PIAF (Fig. 1). The autonomic imbalance and the abrupt loss of central modulation after a GTCS can jointly generate an arrhythmogenic stimulus which would ultimately lead to a paroxysmal AF [5].
In our case, some of the recognized SUDEP risk biomarkers are present. The first is the prolonged PGES pattern, which correlated with a prolonged postictal period. The current criteria for PGES include the immediate postictal (within 30 s), generalized absence of electroencephalographic activity greater than 10 μV in amplitude, allowing for muscle, movement, breathing, and electrode artifact [6]. In the MORTEMUS study, PGES was observed in all the SUDEP patients [7] suggesting that suppression of brain activity related to this pattern is associated with an inhibition of cardiorespiratory activity. As it was demonstrated, GTCS increase the risk of SUDEP [7]. However, this survey evaluated cases studied in EMUs which tends to evaluate patients with focal epilepsy during presurgical evaluation. Drug-resistant genetic generalized epilepsies that occur with tonic–clonic seizures should also be considered at high-risk for SUDEP. In fact, genetic epilepsies associated with specific neuro-cardiac channelopathies such as SCN1A or KCNA1 among others, involve an established SUDEP risk [8]. In our case, the 22q13 deletion syndrome (Phelan–McDermid syndrome) has not been associated with cardiac arrhythmias or sudden death. Although, it is interesting to note that the SHANK3, a gene with limited tissue distribution, is expressed both on cardiac myocytes and neurons. In fact, 25% of patients diagnosed with this syndrome develop epilepsy, although no specific electroclinical syndrome or common interictal EEG findings are associated [9].
AF can trigger cardiac failure with severe hemodynamic repercussions, although in most cases it is asymptomatic and considered a low-mortality arrhythmia. In a retrospective study of SUDEP patients, 56% had ictal-triggered cardiac rhythm or repolarization disturbances, but they showed no differences with the control population. Nonetheless, it was remarkable that two of nine patients presented AF, which is not a frequent seizure-induced arrhythmia [4].
The therapeutic objectives for paroxysmal AF unrelated to epilepsy are the maintenance of sinus rhythm and prevention of peripheral embolization [2]. It is worthwhile considering whether or not PIAF needs to be treated, since seizure control would prevent this arrhythmia. Due to their proarrhythmic effect, the use of sodium-channel blockers should be avoided. Lacosamide can produce a dose-related increase in mean PR interval, and less frequently atrioventricular block. There are a few published cases of AF due to lacosamide, although these cases occurred with high doses and concomitant treatment with other sodium-channel blockers [10], [11]. In our patient, both episodes of AF were recorded after discontinuing lacosamide, so it should not be considered as part of the pathogenic mechanism. When seizure-control is not possible, the indication of antiarrhythmic drugs is controversial, as the pathophysiology of PIAF has not yet been elucidated. Implantation of a pacemaker should only be considered evaluated when symptomatic bradycardia is associated; permanent pacing is not indicated for the prevention of AF in patients without other indications for pacemaker implantation [2]. PIAF has to be considered at the same thromboembolic risk as for every paroxysmal AF unrelated to epilepsy. Therefore the indication of oral anticoagulants should be the same in both cases, according to the personal risk of embolization and hemorrhage [2].
On the other hand, the risk of SUDEP is unclear, nevertheless, as AF is considered a low-mortality arrhythmia, an implantable cardioverter-defibrillator is not generally indicated, although each case should be individually assessed. At least one basal ECG should be performed in every patient with drug-resistant epilepsy and especially prior to a VEM. Also, it is essential to include an ECG-channel in all video-EEG recordings for the detection of peri-ictal cardiac arrhythmias. It is imperative to perform a thorough cardiac evaluation when PIAF is diagnosed, since some of these arrhythmias may be related to structural or arrhythmogenic cardiomyopathy, which are independent risk factors for sudden death. Close observation of patients follow seizures is strongly recommended until normal recovery has occurred. Currently available seizure alert and detection devices may be useful in these cases, however their value remains to be demonstrated in large controlled clinical trials [12].
4. Conclusion
Post-ictal atrial fibrillation is a very infrequent seizure complication. Nevertheless, its real prevalence is probably underdiagnosed since this is a usually short-lasting arrhythmia. This arrhythmia is typically linked with GTCS, possibly in relation to post-ictal generalized electroencephalographic suppression and autonomic dysregulation, and is potentially connected to SUDEP. The use of antiarrhythmic drugs, oral anticoagulants, and cardiac devices (implantable cardiac monitors or implantable cardioverter-defibrillator) should be individualized. We recommend close observation of the patient after every seizure until the patient has fully recovered.
Footnotes
Conflicts of interest: None.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics, Consent, and Permission: Written informed consent was obtained from the patient's family. The publication of this manuscript complied with the Clinical Research Ethics Committee norms of the Hospital del Mar, Barcelona, Spain.
Contributor Information
Alvaro Sanchez-Larsen, Email: aa.sanchezlarsen@gmail.com.
Gemma Aznar-Lain, Email: gaznar@parcdesalutmar.cat.
Begoña Benito, Email: bbenito@parcdesalutmar.cat.
Alessandro Principe, Email: aprincipe@parcdesalutmar.cat.
Miguel Ley, Email: mley@parcdesalutmar.cat.
Adrià Tauste Campo, Email: atauste@imim.es.
Rodrigo Rocamora, Email: rrocamora@parcdesalutmar.cat.
References
- 1.Van der Lende M., Surges R., Sander J.W., Thijs R.D. Cardiac arrhythmias during or after epileptic seizures. J Neurol Neurosurg Psychiatry. 2016;87:69–74. doi: 10.1136/jnnp-2015-310559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.January C.T., Wann L.S., Alpert J.S., Calkins H., Cigarroa J.E., Cleveland J.C. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of cardiology/American heart association task force on practice guidelines and the heart rhythm society. Circulation. 2014;130:e199–e267. doi: 10.1161/CIR.0000000000000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sánchez-Borque P., Rubio J.M., Benezet-Mazuecos J., Quiñones M.A., Farré J. Atrial fibrillation with Wolff-Parkinson-white syndrome in epilepsy: a potentially fatal combination. Seizure. 2015;32:1–3. doi: 10.1016/j.seizure.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Nei M., Ho R.T., Abou-Khalil B.W., Drislane F.W., Liporace J., Romeo A. EEG and ECG in sudden unexplained death in epilepsy. Epilepsia. 2004;45:338–345. doi: 10.1111/j.0013-9580.2004.05503.x. [DOI] [PubMed] [Google Scholar]
- 5.Volders P.G. Novel insights into the role of the sympathetic nervous system in cardiac arrhythmogenesis. Heart Rhythm. 2010;7:1900–1906. doi: 10.1016/j.hrthm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Lhatoo S.D., Faulkner H.J., Dembny K., Trippick K., Johnson C., Bird J.M. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol. 2010;68:787–796. doi: 10.1002/ana.22101. [DOI] [PubMed] [Google Scholar]
- 7.Ryvlin P., Nashef L., Lhatoo S.D., Bateman L.M., Bird J., Bleasel A. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12:966–977. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- 8.Devinsky O., Hesdorffer D.C., Thurman D.J., Lhatoo S., Richerson G. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol. Sep 2016;15(10):1075–1088. doi: 10.1016/S1474-4422(16)30158-2. [DOI] [PubMed] [Google Scholar]
- 9.Phelan K., McDermid H.E. The 22q13.3 deletion syndrome (Phelan–McDermid syndrome) Mol Syndromol. 2012;2:186–201. doi: 10.1159/000334260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degiorgio C.M. Atrial flutter/atrial fibrillation associated with lacosamide for partial seizures. Epilepsy Behav. 2010;18:322–324. doi: 10.1016/j.yebeh.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 11.Shaibani A., Fares S., Selam J.L., Arslanian A., Simpson J., Sen D. Lacosamide in painful diabetic neuropathy: an 18-week double-blind placebo-controlled trial. J Pain. 2009;10:818–828. doi: 10.1016/j.jpain.2009.01.322. [DOI] [PubMed] [Google Scholar]
- 12.Langan Y., Nashef L., Sander J.W. Case-control study of SUDEP. Neurology. 2005;64:1131–1133. doi: 10.1212/01.WNL.0000156352.61328.CB. [DOI] [PubMed] [Google Scholar]