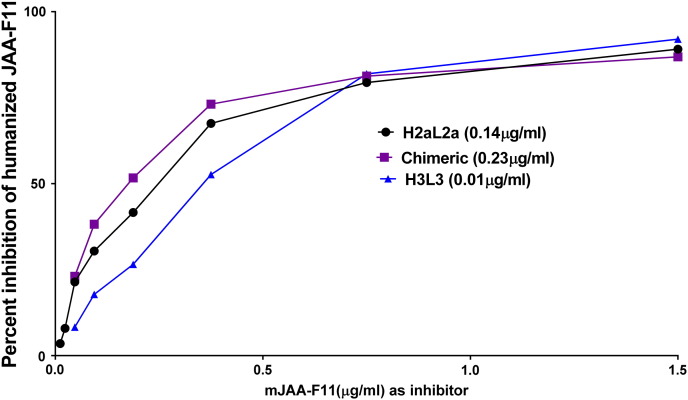
Figure 4.
The humanized H3L3 has a higher affinity than H2aL2a antibody which has a higher affinity than chimeric antibody to TF-Ag-BSA. A) Graph shows relative affinity of H2aL2a and chimeric antibodies to TF-Ag as determined by ELISA. Each antibody was mixed with serial dilutions of mJAA-F11 and binding to the TF-Ag coated plate was measured by using a species-specific alkaline phosphatase anti-human IgG antibody. The amount of mJAA-F11 antibody required to inhibit 1 μg of each tested antibody (H3L3, H2aL2a, and chimeric antibodies) to 50% was extrapolated and is depicted in the table (B). The higher the amount of mouse antibody required for inhibition, the higher the relative affinity of the antibody. ANOVA analysis was performed on the relative affinity (IC50) values obtained for each antibody tested (as described above) and binding of H3L3 was significant at P < .001 (****). Humanized and chimeric antibodies used at a concentration which would yield an O.D. of 1.00 in the EIA when not inhibited. H3L3 used at ~10 times the H2aL2a concentration and ~15 times the chimeric concentration.