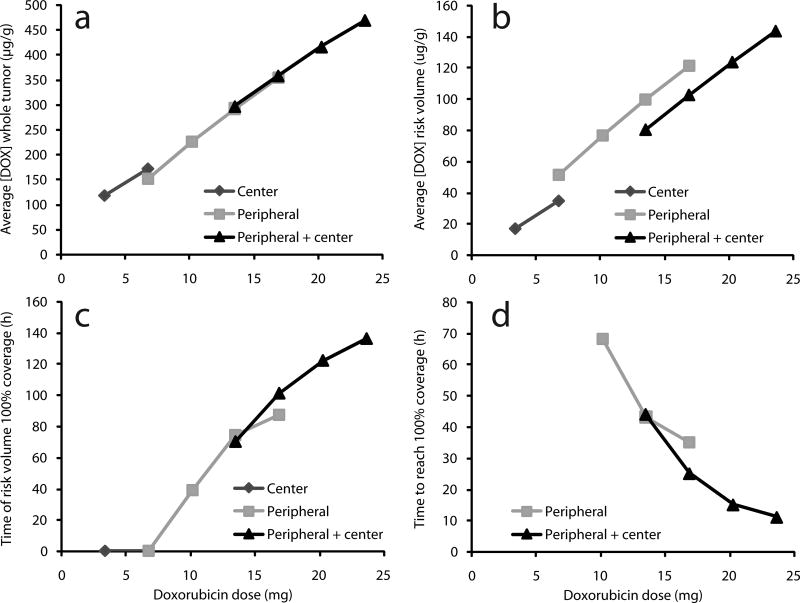
Figure 4.
Characteristics of simulated drug distribution with central implant, peripheral implants, or peripheral+central implants as a function of total DOX dose. Average doxorubicin concentration [DOX] over 8 days in (a) the entire tumor or (b) outer tumor rim (risk volume). (c) Simulated duration for which [DOX] ≥ 12.8 µg/g in entire risk volume. (d) Time after implantation at which [DOX] ≥ 12.8 µg/g in entire risk volume.