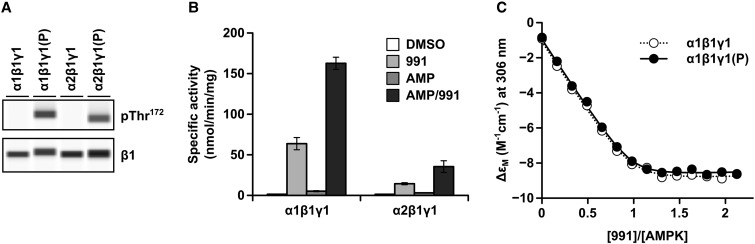
Figure 1. Allosteric activation of non-phosphorylated Thr172 AMPK.
(A) AMPK α1β1γ1 and α2β1γ1 complexes were expressed in E. coli, purified and analysed by Western blotting using either an anti-pThr172 antibody or an anti-β antibody. Recombinant complexes that had been phosphorylated with CaMKKβ [α1β1 γ1(P) and α2β1γ1(P)] are shown alongside the non-phosphorylated proteins. (B) AMPK activity of the non-phosphorylated complexes was measured using the SAMS peptide assay in the absence or presence of 991 (1 µM), AMP (10 µM) or both 991 and AMP. Results shown are plotted as nmol/min/mg and are the means ± SEM of three independent experiments. (C) Comparison of circular dichroism signal change measured at 306 nm for non-pThr172 (open symbols) and pThr172 (closed symbols) α1β1γ1 complexes as a function of 991/AMPK.