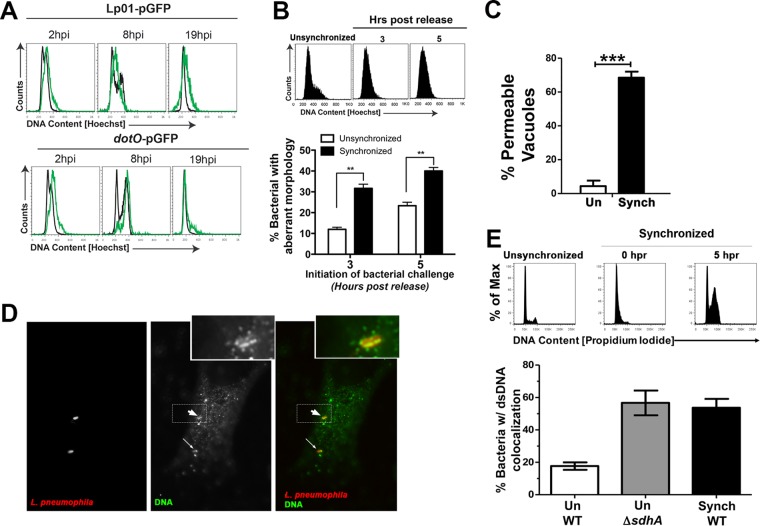
FIG 7 .
L. pneumophila cell cycle arrest in S phase results in loss of vacuole integrity. (A) HeLa cells were synchronized by the double-thymidine block method and challenged with wild-type L. pneumophila/pGFP (Lp01) or the ΔdotO/pGFP mutant 3 h after release. Various times after uptake, cells were collected and analyzed by flow cytometry to determine the cell cycle profile of Hoechst-stained cells. Infected cells were separated from within the total population based on GFP fluorescence. Black lines indicate uninfected cells and green lines infected cells. (B) Challenge of S phase cells with L. pneumophila results in bacterial degradation. HeLa cells were synchronized by double-thymidine block and challenged with L. pneumophila Lp01 3 or 5 h after release. (Top panel) Hoechst staining of cells in the absence of synchronization or at noted times of release. The infection was then allowed to proceed for 6 h, followed by fixation and staining with anti-L. pneumophila. (Bottom panel) The number of bacteria with aberrant morphology was scored visually as in Fig. 6D (Materials and Methods). (C) Challenge of S phase cells results in permeable LCVs. HeLa cells were synchronized as in panel B, and the cells were released for 3 h prior to challenge with Lp01. At 6 h postinfection, the cells were fixed, and vacuole integrity was determined by probing with anti-L. pneumophila in the absence of chemical permeabilization (Materials and Methods). (D) Detection of bacterium-associated DNA in S phase cells. Synchronization was performed as in panel B, and cells were challenged with Lp01 5 h postrelease. Six hours later, the samples were fixed, probed with anti-DNA (green), permeabilized, and then probed with anti-L. pneumophila (red) and analyzed by immunofluorescence microscopy. The dashed box represents area that is magnified in the inset, with the fat arrow pointing to an internalized bacterium. Insets were artificially magnified by a 4.167-fold increase in the pixel density. The thin arrow points to a second internalized bacterium. (E) Challenge of S phase cells with L. pneumophila results in exposure of bacterium-associated DNA. Cells synchronized by double-thymidine block were released for 5 h (top panel), challenged for 6 h, and probed as in panel D, and the fraction of bacteria showing DNA association was determined by immunofluorescence microscopy (bottom panel). Un WT, unsynchronized cells challenged with WT; Un ΔsdhA, unsynchronized cells challenged with the ΔsdhA mutant; Synch WT, synchronized cells challenged 5 h after release with the WT.