Following the introduction of imatinib and several other tyrosine kinase inhibitors (TKIs), the clinical scenario of Philadelphia chromosome-positive (Ph+) and BCR-ABL1-positive (BCR-ABL1+) chronic myeloid leukemia (CML) has changed almost completely.1 Although the number of studies is limited, and the follow up is short and sometimes defective, survival data are substantial; around 90% at 5 years, 89% at 6 years, 86% at 8 years, and 83–84% at 10 years2–10 (Table 1). Only 50% of deaths are due to the progression of leukemia, while 50% occur in remission and are due to other causes which occasionally include treatment-related toxicity and related complications. Relative survival analyses have concluded that the life expectancy of a patient with chronic phase (CP) CML, under proper TKI treatment, is now very close or almost identical to the life expectancy of non-leukemic, age-matched individuals.11,12 New drugs, both TKIs and non-TKIs, are emerging. New, more sophisticated molecular technologies are available. So, which problems remain to be solved? Is there still room for improvement, and where?
Table 1.
A summary of the outcome of treatment with TKIs of newly diagnosed CP CML patients. Only the studies reporting on more than 200 patients, with a median follow-up observation of longer than 5 years, are listed. Notice the important differences in age and in the proportion of high-risk patients. MD Anderson, German CML IV and GIMEMA are academic studies. The Swedish data are taken from a population-based registry. IRIS, ENESTnd and DASISION are company-sponsored, registrative studies
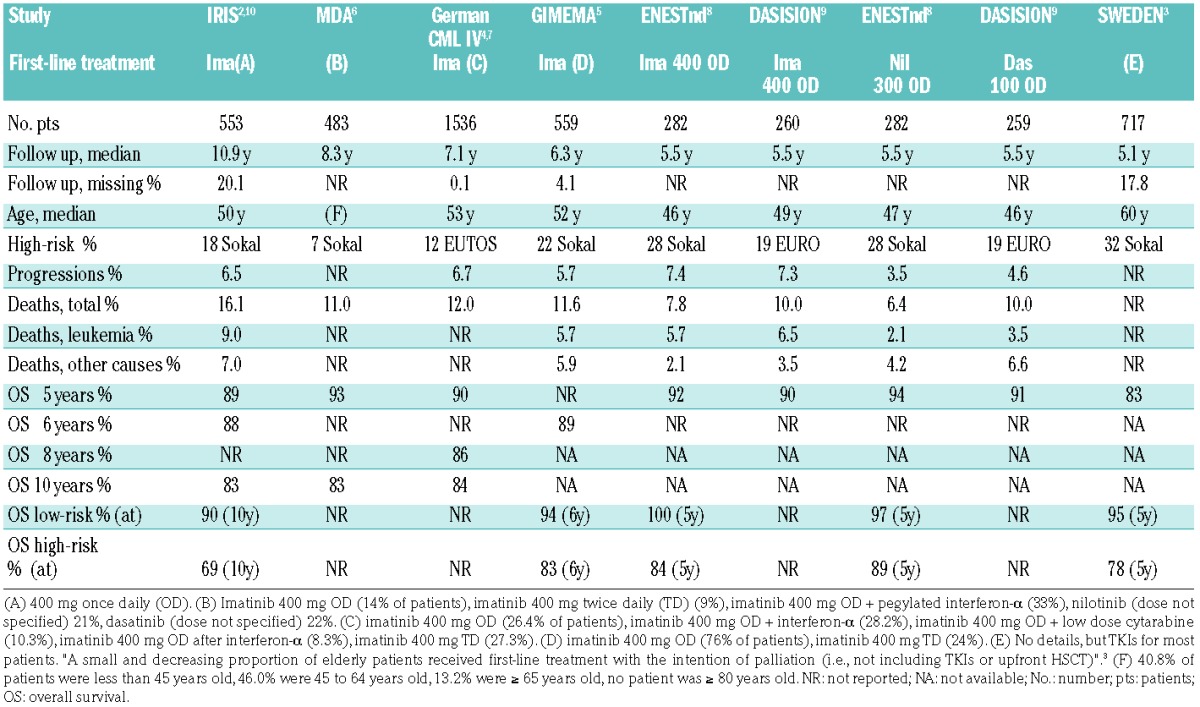
There is still room for improvement for patients with newly diagnosed accelerated phase (AP) and blastic phase (BP) CML, and to some extent also for patients with high-risk CP CML. The former account for 4% to 5% of all newly diagnosed patients.13 They respond to the TKI treatment, but the extent of response is inferior, and their ultimate outcome is still unclear.1 High-risk patients account for 10% to 25% of newly diagnosed CP CML patients, depending on which risk score is used, but even using Sokal, which is less selective and includes many more patients than Euro, European Treatment Outcome Study (EUTOS), and the new EUTOS long-term survival score,14 the outcome of these patients is inferior, with a reported survival of 83%–89% at 5–6 years and of 68% at 10 years7,10 (Table 1). In addition, the presence of clonal chromosome abnormalities in Ph+ cells (CCA/Ph+) at baseline, which occurs in 3% to 4% of patients, is a marker of an inferior outcome.1 It is believed that all these patients (AP, BP, high-risk CP, CCA/Ph+) can benefit more from second- or third-generation TKIs, and that some of them, in particular those of younger age, are candidates for allogeneic stem cell transplantation. However, much needed prospective, specifically addressed trials are lacking.
But is there still room for improvement for the 80% to 90% of patients who become optimal responders and have a normal life expectancy? TKIs cannot prolong life, but their proper use can help to improve the quality of life without precluding the achievement of a remission that remains stable even after treatment discontinuation (treatment-free remission, TFR). Concerning the quality of life, many studies report that the side effects or toxic effects of TKIs were “manageable”, could be tolerated, and did not impel a change of TKI. Typically, such studies were designed to limit the switch from one TKI to another, and were analyzed more to define the tolerability profile of a specific TKI than to assess the quality of life of the patients. We suggest that in the case of so-called “manageable” side effects, mild or recurrent, that impair the daily life of a patient, more consideration should be given to a change of the drug and also to a change of the dose, provided that an optimal response is maintained. At times a patient-adapted policy, that is a policy adapted to side effects and to response, can be more convenient. For that purpose, trials may not be necessary, but a careful long-term observation will be important to control overall how patients adapt to side effects and to pick up on the so-called unexpected adverse events.
TFR is an acronym that fully expresses the success of therapy, combining the perception of cure, a normal life expectancy, no treatment-related side effects and complications, independence from drugs, in addition to a proper use of the financial resources of health systems that are more and more challenged by the introduction of new and effective, but also expensive drugs.15–17 It is expected that a more widespread use of second-generation TKIs in first-line treatment would result in increased TFR. However, evidence is still missing as there are no studies reporting on the benefit (the TFR rate) and the cost (toxicity) of a policy of imatinib use vs. a policy of the use of second-generation TKIs, either in early or late first- or second-line therapy.17 Such studies require time, patience and resources, but are necessary in order to move from expectation to evidence. Gruppo Italiano Malattie EMatologiche dell’Adulto (GIMEMA) and the Haemato Oncology Foundation for Adults in the Netherlands (HOVON) are currently running a trial of first-line nilotinib vs. first-line imatinib therapy with a switch to nilotinib in the event of less than optimal response, with the TFR rate at 5 years being the primary endpoint.18 More than five years will be required to obtain answers from this study. Furthermore, the choice between continuing treatment indefinitely and no treatment at all may be challenged and alternative policies are currently being tested, either of partial, intermittent treatment,19,20 or of graded discontinuation.21 For the time being we must acknowledge that any recommendation concerning the policy of treatment regarding TFR is not yet evidence-based. In all likelihood, the best policy does not exist, and different policies may be successful in different situations.
Notwithstanding the fact that the treatment and the modalities of treatment for TFR are still an issue for research, it should not be overlooked that treatment discontinuation and TFR are already a reality in practice, and are the goal of more and more patients. There are solid data showing that treatment discontinuation after five or more years of TKIs, and after one or more years of deep molecular response (MR), such as MR 4.0 (BCR-ABL1 ≤ 0.01% on the international scale), and particularly MR 4.5 (BCR-ABL1 ≤ 0.0032%, on the international scale) results in a rate of TFR of 50% or more, and that in the event of molecular relapse the resumption of treatment brings all patients back to molecular remission.15,16,22,23
In summary, once a deep MR is achieved, and provided that careful molecular monitoring is assured, no patient will die of leukemia because of treatment discontinuation, and 50% will enjoy a treatment-free life. Therefore, we suggest that both the possibilities and the problems of treatment discontinuation should be discussed not only with all the patients who fit the current, provisional eligibility criteria for discontinuation, but also with newly diagnosed patients.
Supplementary Material
References
- 1.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia. Blood. 2013;2013;122(6):872–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. [DOI] [PubMed] [Google Scholar]
- 3.Hoglund M, Sandin F, Hellstrom K, et al. Tyrosine kinase inhibitor usage, treatment outcome, and prognostic scores in CML: report from the population-based Swedish CML registry. Blood. 2013;122(7):1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hehlmann R, Muller MC, Lauseker M, et al. Deep molecular response is reached by the majority of patients treated with imatinib, predicts survival, and is achieved more quickly by optimized high-dose imatinib: results from the randomized CML-Study IV. J Clin Oncol. 2014;32(5):415–423. [DOI] [PubMed] [Google Scholar]
- 5.Castagnetti F, Gugliotta G, Breccia M, et al. Long-term outcome of chronic myeloid leukemia patients treated frontline with imatinib. Leukemia. 2015;29:1823–1831. [DOI] [PubMed] [Google Scholar]
- 6.Jain P, Kantarjian H, Alettar ML, et al. Long-term molecular and cytogenetic response and survival outcomes with imatinib 400 mg, imatinib 800 mg, dasatinib, and nilotinib in patients with chronic-phase chronic myeloid leukaemia: retrospective analysis of patients data from five clinical trials. Lancet Haematol. 2015;2(3):e118–e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalmanti L, Saussele S, Lauseker M, et al. Safety and efficacy of imatinib in CML over a period of 10 years: data from the randomized CML-Study IV. Leukemia. 2015;29(5):1123–1132. [DOI] [PubMed] [Google Scholar]
- 8.Hochhaus A, Saglio G, Hughes T, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30(5):1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the dasatinib versus imatinib study in treatment-naive chronic myeloid leukemia patients trial. J Clin Oncol. 2016;34(20):2333–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochhaus A, Larson RA, Guilhot F, et al. IRIS final analysis: long-term outcomes with imatinib treatment for CML. N Engl J Med. 2017, submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasaki K, Strom SS, O’Brien S, et al. Relative survival in patients with chronic phase chronic myeloid leukaemia in the tyrosine-kinase inhibitor era: analysis of patients data from six prospective clinical trials. Lancet Haematol. 2015;2(5):e186–e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bower H, Bjorkholm M, Dickman PW, Hoglund M, Lambert PC, Andersson TML. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol. 2016;34(24):2851–2857. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann VS, Baccarani M, Hasford J, et al. The EUTOS population-based registry: incidence and clinical characteristics of 2904 CML patients in 20 European countries. Leukemia. 2015;29(6):1336–1343. [DOI] [PubMed] [Google Scholar]
- 14.Pfirrmann M, Baccarani M, Saussele S, et al. Prognosis of long-term survival considering disease-specific death in patients with chronic myeloid leukemia. Leukemia. 2016;30(1):48–56. [DOI] [PubMed] [Google Scholar]
- 15.Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128(1):17–23. [DOI] [PubMed] [Google Scholar]
- 16.Saussele S, Richter J, Hochhaus A, Mahon F-X. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. 2016;30(8):1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baccarani M. Treatment-free remission in chronic myeloid leukemia: floating between expectation and evidence. Leukemia. 2017;31(4):1015–1016. [DOI] [PubMed] [Google Scholar]
- 18.SUSTRENIM (Sustained treatment-free remission in BCR-ABL+ chronic myeloid leukemia: a prospective study comparing nilotinib versus imatinib with switch to nilotinib in absence of optimal response). ClinicalTrials.gov: NCT02602314.
- 19.Russo D, Martinelli G, Malagola M, et al. Effects and outcome of a policy of intermittent imatinib treatment in elderly patients with chronic myeloid leukemia. Blood. 2013;121(26):5138–5144. [DOI] [PubMed] [Google Scholar]
- 20.OPTkIMA (Phase-III randomized study to optimize TKIs multiple approaches and quality of life in elderly patients with Ph+ chronic myeloid leukemia and MR 3.0 / MR 4.0 stable molecular response). ClinicalTrials.gov:NCT02326311.
- 21.Clark R, Polydoros F, Apperley JF, et al. Chronic myeloid leukaemia patients with stable molecular responses (at least MR3) may safely decrease the dose of their tyrosine kinase inhibitor: data from the British Destiny study. Blood. 2016;128(22):938. [Google Scholar]
- 22.Mahon F-X, Richter J, Guilhot J, et al. Cessation of tyrosine kinase inhibitors treatment in chronic myeloid leukemia patients with deep molecular response: results of the Euro-Ski trial. Blood. 2016;128 (22):787. [Google Scholar]
- 23.Etienne G, Guilhot J, Rea D, et al. Long-term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35(3):298–305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


