Figure 1.
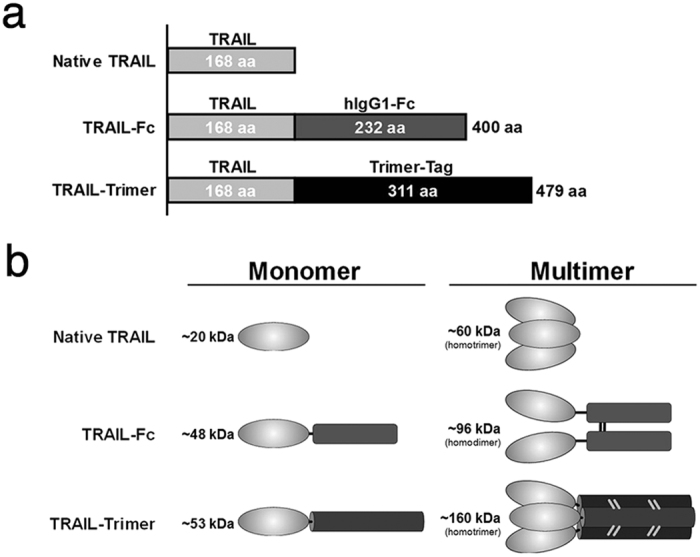
Structures of different TRAIL polypeptides. (a) Three recombinant soluble forms of human TRAIL have been used in this study: a native TRAIL comprised of the extracellular domain of TRAIL, a dimeric TRAIL-Fc comprised of extracellular TRAIL domain fused to human IgG1 Fc domain, and a TRAIL-Trimer comprised of extracellular TRAIL domain fused to Trimer-Tag. Amino acid sequence lengths are shown for each protein and domain respectively. (b) Theoretical molecular weights (kDa) of both monomeric and multimeric forms of native TRAIL, TRAIL-Fc and TRAIL-Trimer, respectively. Native TRAIL associates into a non-covalently-linked homotrimer, TRAIL-Fc forms a covalently-linked homodimer, and TRAIL-Trimer forms a covalently-linked homotrimer.
