Abstract
Study Objectives:
Little is known about the type and severity of sleep disturbances in the pediatric chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) population, compared with healthy adolescents. Using a range of objective and subjective measures, the aim of this study was to investigate sleep quality, the relationship between objective and subjective measures of sleep quality, and their associations with anxiety in adolescents with CFS/ME compared with healthy controls.
Methods:
Twenty-one adolescents with CFS/ME aged 13 to 18 years (mean age 15.57 ± 1.40), and 145 healthy adolescents aged 13 to 18 years (mean age 16.2 ± 1.00) wore actigraphy watches continuously for 2 weeks to collect a number of objective sleep variables. The Pittsburgh Sleep Quality Index was used to obtain a subjective measure of sleep quality. Anxiety was measured by the Spence Children's Anxiety scale.
Results:
On average over the 2-week period, adolescents with CFS/ME were found to have (1) significantly longer objective sleep onset latency, time in bed, total sleep time, and a later rise time (all P < .005), and (2) significantly poorer subjective sleep quality (P < .001), compared with healthy adolescents. The CFS/ME patient group displayed higher levels of anxiety (P < .05), and in both groups, higher levels of anxiety were significantly related to poorer subjective sleep quality (P < .001).
Conclusions:
This study provides objective and subjective evidence of sleep disturbance in adolescents with CFS/ME compared with healthy adolescent controls.
Citation:
Josev EK, Jackson ML, Bei B, Trinder J, Harvey A, Clarke C, Snodgrass K, Scheinberg A, Knight SJ. Sleep quality in adolescents with chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J Clin Sleep Med. 2017;13(9):1057–1066.
Keywords: actigraphy, adolescent, chronic fatigue syndrome, myalgic encephalomyelitis, PSQI, sleep diary, sleep quality
INTRODUCTION
Persistent, excessive, and debilitating fatigue is a primary feature of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). In addition, CFS/ME is characterized by sleep disturbance, postexertional malaise, and impairments in cognitive, autonomic, neuroendocrine, and/or immune functioning.1 Although extensive literature exists investigating the illness in adults, more recently, research has focused on how this chronic condition affects the pediatric population, with prevalence estimated to be within the 0.001% to 2% range2 for this younger group.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Little is known about the type and severity of sleep disturbances in the pediatric CFS/ME population, particularly in comparison with the healthy adolescent population. Poor or disordered sleep can affect future educational, cognitive, psychosocial, psychopathological, and physical health outcomes, and may exacerbate the impact of other CFS/ME symptoms.
Study Impact: This study provides objective and subjective evidence of sleep disturbances in adolescents with CFS/ME compared with healthy adolescents. Psychological factors such as anxiety do not appear to contribute to objective sleep disturbance, but may play a role in influencing adolescents' perception of their sleep quality. Both objective and subjective factors may be important for clinicians to consider when treating adolescent patients with sleep disturbance.
The typical onset of pediatric CFS/ME in middle to late adolescence occurs during an important period of academic, social, emotional, hormonal, physical, and brain development.3–6 Importantly, associated maturational changes in the quality of sleep (ie, duration, timing, efficiency) also occur in the transition from childhood to adolescence.7–11 For instance, healthy adolescents are observed to demonstrate adaptation of their sleep-wake cycle to the environment, including an overall reduction in total sleep time, later bedtimes, and an increase in sleep onset latency.7,12 However, relatively little is known about the type and severity of sleep disturbances in the adolescent CFS/ME population, and how this compares to the quality of sleep experienced by the healthy adolescent population. This is pertinent given that poor or disordered sleep can affect future educational and cognitive outcomes,13 psychosocial and psychopathological outcomes,14 and physical health15 in children and adolescents, and therefore may exacerbate the effect of other CFS/ME symptoms. A more comprehensive understanding of sleep disturbances in adolescents with CFS/ME may also facilitate targeted research on effective sleep interventions for this population. Importantly, investigation of both objective measures of sleep quality (ie, actigraphy or polysomnography) and subjective measures (ie, self-report questionnaires, sleep-wake diaries) is recommended as complementary sources for characterizing sleep disturbances in adults16 and children.17 Subjective measures are often inexpensive, easily administered, and provide detailed descriptions of sleep characteristics, whereas objective measures such as actigraphy can provide additional impartial information about sleep quality.17
A recent systematic review12 found that, since 1987 (ie, the year in which the official diagnosis of CFS/ME was first published), only 6 studies have investigated sleep in children and adolescents younger than 19 years with a diagnosis of CFS/ ME. The studies are characterized by wide variation with respect to the inclusion criteria employed (ie, some have used different case definitions of CFS/ME), sample size (ie, ranging from n = 3 to n = 57),12 method used to assess sleep (ie, actigraphy, polysomnography, core body temperature, sleep/wake diary), sleep outcomes (ie, total sleep time, sleep efficiency, sleep latency), time frame of data collection (ie, polysomnography for 1 night, actigraphy for 2 weeks), and the investigation of biological factors that potentially underlie sleep disturbances (ie, core body temperature, cortisol levels, melatonin levels).
Increased objective sleep disturbance in participants with CFS/ME in comparison with healthy controls was a common finding in previous studies.12 Compared with controls, children and adolescents with CFS/ME showed significantly longer total sleep time,18 more disrupted sleep and reduced sleep efficiency,18,19 reduced rapid eye movement (REM) and non-REM stage 2 sleep,20 delayed time of appearance of the lowest core body temperature and lower amplitude of changes in circadian core body temperature,21 higher levels of melatonin in the first part of the night and early morning,22 and temporal differences in bouts of actual rest during time in bed.19 Nevertheless, children and adolescents with CFS/ME have also been shown to have objective sleep parameters that are comparable to healthy controls such as actual sleep time,20,22 sleep onset latency,20,22 REM latency, and the amount of non-REM stage 1 sleep.20 The inconsistency in findings may relate to the variable methodologies and measures employed. For instance, of the 6 studies in the recent systematic review,12 2 studies used actigraphy, 2 studies used polysomnography, and 2 studies used a sleep/wake diary. Importantly, the use of actigraphy in pediatric CFS/ME studies is relatively rare, despite it being recommended as a more ecologically valid and sensitive measure of sleep quality and routine because it is performed in a naturalistic setting (ie, in the individual's home).23,24 The small sample sizes in these studies may also account for the inconsistency in findings.
Only one previous study has investigated sleep on a subjective basis in pediatric CFS/ME. Subjective measures are important because they are often a more economically and methodologically feasible way of measuring sleep quality.25 They provide valuable information about the patient's sleep experience, which has the potential to influence objective measurements. Knook and colleagues found that children and adolescents with CFS/ME reported more unrefreshing sleep, greater restless sleep, and more nocturnal wake-ups than healthy controls.22 This is in accord with studies that have reviewed medical records of pediatric CFS/ME patients, where other than fatigue, sleep disturbance is one of the most commonly reported symptoms of the condition.26,27 Of interest, the direct association between self-report of unrefreshing sleep and objective measures of sleep quality remains to be investigated in the pediatric CFS/ME population. In adult patients with CFS/ME, objective measures of sleepiness (ie, the Multiple Sleep Latency Test) have been shown not to relate to subjective measures of sleepiness (ie, Stanford Sleepiness Scale), which suggests that the unrefreshed sleep experienced by this population when measured objectively may be distinct from their self-report of sleep quality.23 Furthermore, research from Le Bon and colleagues suggests that although primary sleep disorders are a relatively common comorbidity for adult patients with CFS/ME, CFS/ME symptomatology is distinct from primary sleep disorders and does not merely represent the somatic expression of sleepiness.28 Therefore, it is important to investigate both objective and subjective reports of sleep quality within the same population. Of note, none of the 6 pediatric CFS/ME studies conducted to date have comprehensively investigated both an objective and validated subjective (self-report) measure of sleep disturbance.12
In addition, it is also worth investigating other factors that may be associated with sleep quality such as demographic characteristics (ie, age, sex) and illness characteristics (ie, illness duration, factors that trigger illness, comorbid psychopathology) because these may aid in the identification of individuals with CFS/ME who are especially vulnerable to sleep disturbance. Snodgrass and colleagues reported that no previous study had investigated these characteristics in a pediatric CFS/ ME cohort, despite previous research suggesting that they may play a role in other chronic conditions.12,29,30 For instance, self-reported variations in presleep rumination have been shown to predict an increase in actigraphy-measured sleep onset latency31 suggesting that anxiety-related symptomatology may influence sleep quality in some individuals. Indeed, Knight and colleagues found that 17% of adolescents with CFS/ME had comorbid anxiety based on a retrospective review of medical records, although rates are estimated to be as high as 50% when based on diagnostic interviews and self-report questionnaires.27 It is therefore critical that psychological factors such as anxiety are measured in a standardized manner to assess their association with sleep quality.
Overall, previous studies provide some suggestion that adolescents with CFS/ME may experience disturbance in the quality of sleep compared with their healthy peers, which is apparent on both subjective and objective measures. However, research into pediatric CFS/ME sleep disturbance is currently limited by a paucity of studies, inconsistent findings, variable methodologies, a lack of standardized measures and reporting of important illness characteristics, and a lack of investigation of other factors that may influence sleep quality (ie, illness duration, age, pain, comorbid mood disturbance),12 which restricts our ability to interpret these results within the wider clinical context.
The current study employed both objective and standardized subjective measures to examine sleep. The primary aim of the current study was to investigate overall sleep quality in adolescents in whom CFS/ME is diagnosed, compared with healthy adolescent controls. It was hypothesized that adolescents with CFS/ME would have poorer sleep quality than healthy adolescent controls on a range of objective measures (ie, actigraphy) and subjective measures (ie, the Pittsburgh Sleep Quality Index [PSQI]). The second aim was to investigate the association between anxiety and sleep quality in adolescents with CFS/ME and in healthy adolescent controls. Adolescents with CFS/ME were hypothesized to show greater levels of anxiety compared to controls, and level of anxiety was hypothesized to relate to sleep quality in both groups, such that poorer sleep quality would be associated with greater anxiety. Finally, the third aim was to examine the relationships between objective and subjective measures of sleep quality.
METHODS
Participants
To be included in the study, patients had to: (1) be aged 13 to 18 years, (2) present to the Royal Children's Hospital (RCH) CFS/ME Clinic for the first time between December 2013 and March 2015, and (3) receive a diagnosis of CFS/ME at the RCH CFS/ME Clinic by their treating pediatrician, according to the pediatric case definition32 (ie, persistent or recurring fatigue for at least 3 months and concurrent occurrence of (a) postexertional malaise, (b) unrefreshing or disturbed sleep, (c) myofascial and/or joint pain, or abdominal and/or head pain, (d) two or more neurocognitive manifestations, (e) at least one symptom from two of the following categories: autonomic, neuro-endocrine, immune, and (f) the exclusion of any active medical or psychiatric condition that may explain the presence of chronic fatigue). Primary complaint of sleep disturbance at the time of study enrollment was not a required inclusion criterion for the current study. Patients were excluded if their English language skills were insufficient to complete the questionnaires or if the diagnosis of CFS/ME was unclear.
The control sample was recruited from Australian secondary colleges via flyers in community centers and e-newsletters through participating schools, as part of a larger adolescent sleep study at the Melbourne School of Psychological Sciences at the University of Melbourne, and has been described previously.33 Two movie vouchers were provided as an incentive. Control participants were aged 13 to 18 years, all were attending school (100%), and a small proportion were included who had psychiatric (6.16%) and sleep conditions (11.64%). One participant was excluded because of the presence of multiple sleep disorders, one due to lost actigraphy, and one due to physical illness in the first week. Other than these exceptions, no control participants reported suffering from mental or physical conditions, or using sleep aids or medication such as melatonin.
Invitations to participate, information statements, and consent forms were sent by mail. Informed consent was obtained from all teenage participants and their parents. CFS/ME participants were not offered any compensation or incentives to participate in the research. This study was approved by The RCH Human Research Ethics Committee and the Human Research Ethics Committee of the University of Melbourne.
Measures
Actigraphy Watches
Actigraphy watches (Actiwatch 2, Philips Respironics, Sydney, Australia, Koninklijke Philips N.V., 2008) were used to obtain objective measures of sleep quality. The watches recorded motion and light exposure continuously in 1-minute intervals on individual actograms. Actiware software (version 6, Philips Respironics, Koninklijke Philips N.V.) was used to analyze the data based on the software's “medium” sleep/wake threshold, after which further visual screening of individual actograms was performed. Visual screening has been shown to have a greater correlation than machine-automated algorithms in the assessment of actograms.34 Variables generated by the Actiware software were sleep onset latency (SOL) in minutes, sleep efficiency (SE) as a percentage (ie, total sleep time as a percentage of time in bed), percentage of wake after sleep onset (%WASO), time in bed (TIB) in minutes, total sleep time (TST) in minutes, rise time (RT), in minutes past midnight and bed time (BT) in minutes past 6:00 AM. For the purpose of this study, only nocturnal sleep patterns on school nights (Monday to Thursday during school term) were analyzed. Average values were obtained over the 2-week period for both groups. School nights were chosen for analysis to maximize consistency when comparing the CFS/ME group and control group, and to limit confounding factors such as changed routines on Friday nights and weekends. It is well recognized that Friday nights and weekends have distorting effects on sleep patterns in adolescents (ie, adolescents typically stay up later in the evening and wake up later in the morning35–37), and as such, these days were valued as “transitional” days and not included in the recordings.
Wake and Sleep Diary
For the CFS/ME group only, a researcher-designed wake and sleep diary (a short 5-minute questionnaire) adapted for adolescents38,39 was also used regarding sleep (completed by participants each morning upon waking) and daytime activity (completed each night prior to their bed time), including questions about their total TIB, SOL, TST, BT, and RT. Although not used directly for analysis, these data were used for visual assessment of individual actigraphy watch data. Within the diary, participants also recorded the times when the watch was removed (ie, for bathing or swimming) so these could be excluded from the individual actigraphy watch data. Diary data used for analysis included time spent using an electronic device (ie, computer, phone, tablet), and whether sleeping aids were taken (ie, lavender, melatonin, prescription sleeping tablet). Of note, in the RCH CFS/ME clinic, patients were provided with general sleep hygiene advice and were prescribed melatonin as appropriate (melatonin is a prescription medication in Australia), yet doses and time periods of use for these treatments (or any other medications) were not recorded in the current study. Although sleep hygiene advice represents a standard of care for patients with CFS/ME in Australia (see RACP Clinical Practice Guidelines40), melatonin is not clearly recommended in these guidelines and the frequency of melatonin use in adolescents with CFS/ME has not been documented. To match the actigraphy data, only data from Monday to Thursday during the school term were analyzed, and averaged over the 2-week period.
Pittsburgh Sleep Quality Index
The self-rated PSQI was used to obtain a subjective measure of sleep quality. The PSQI measures 7 main domains of sleep (each of which has a range of 0–3 points, where 0 indicates no difficulty sleeping, and a score of 3 indicates severe difficulty sleeping) with 19 individual questions, based on an individual's sleep patterns during the previous month.41 The 7 component scores are added to generate the global PSQI score (ranging from 0 to 21 points), with a higher score indicating severe sleep difficulties in multiple domains. A global score above 5 is indicative of a “poor sleeper.” The PSQI has been shown to have good psychometric properties as well as internal validity and consistency in adult populations (ie, Cronbach alpha of .8)42,43 and adolescent populations (ie, Cronbach alpha of .72).44 The PSQI was completed by participants prior to commencing the actigraphy watch data collection, with the global score used to measure subjective self-reported sleep quality.
Spence Children's Anxiety Scale
The Spence Children's Anxiety Scale (SCAS),45 a child and adolescent self-report mood measure, was used to assess symptoms of anxiety (ie, separation anxiety, social phobia, obsessive-compulsive disorder, panic-agoraphobia, generalized anxiety, and fears of physical injury). Participants were asked to rate on a 4-point scale from 0 to 3 points: “never,” “sometimes,” “often,” or “always.” The SCAS global z score for “total anxiety” score was used for the current study, which yielded a maximum possible score of 114. The SCAS is a well-validated measure with high reliability and internal consistency (ie, Cronbach alpha between .6 and .8), with normative data in Australian adolescents.46
Parent Questionnaire
Parents of the CFS/ME group also completed a brief questionnaire to obtain illness characteristics including illness duration, factors that trigger illness, and school attendance (ie, the proportion of full-time schooling attended).
Procedure
Each participant received an Actiwatch either in person or by mail, along with clear verbal and written instructions on how to use the watch. An email link to the questionnaires (ie, PSQI, SCAS, parent questionnaire) was emailed to the participant's nominated email, which was filled out prior to starting the Actiwatch data collection, and completed through REDCap Software (version 5.10.2, 2014, Vanderbilt University, Nashville, Tennessee, United States), a secure, web-based application. Hyperlinks to individual wake and sleep diaries were also emailed every morning (5:30 AM, sleep diary) and night (7:00 PM, wake diary) during the 2-week period. Participants were instructed to wear the watch continuously over a 2-week period of school days during the school term (Monday to Thursday) on the nondominant wrist, but were allowed to remove the watch for bathing and swimming. The participants were asked to press the event marker button on the side of the watch when going to sleep and upon waking from sleep (BT and RT, respectively). After participants completed a full 2-week period of wearing the Actiwatch, they were required to mail the watch back to the RCH in a reply paid envelope.
Statistical Analyses
The statistical analysis program Stata 13.0 (StataCorp, 2013. Stata Statistical Software: Release 13. College Station, Texas, United States: StatCorp LP) was used for data analysis. Some participants in the CFS/ME group had incomplete/missing data for school attendance (n = 2), SCAS (n = 3), and the PSQI (n = 5), and Actiwatch activity (n = 1, technical fault). In the control group, a small number of participants had incomplete/ missing data on the SCAS and PSQI (n = 6), and Actiwatch activity (n = 5, technical faults and noncompliance; see Bei et al.33). No actigraphy data were lost due to user error.
Preliminary analyses included chi-square, Mann-Whitney U, and independent samples t tests for group differences, and univariate regression analyses were conducted on the associations between individual characteristics such as illness duration, school attendance, and time spent using an electronic device, and objective and subjective sleep measures.
Given the small sample of CFS/ME patients, the study was underpowered to perform age- and sex-matched comparisons between the patient and control groups. Therefore, for the first aim, separate linear regression analyses were conducted to evaluate group differences in objective sleep quality, where each individual sleep quality variable was the outcome (ie, SOL, SE, PSQI global scores, etc.) and “group” was the predictor, adjusting for either sex or age where necessary (ie, if group differences were found in sex or age). Mean differences (ie, unstandardized regression coefficient [b]) with P values were reported. Given the multiple comparisons made in this study, standardized regression coefficients (β) were also evaluated, without relying solely on the P values. The β coefficients were used as measures of the magnitude of effect (cutoff points: .1 indicative of weak prediction, .3 indicative of moderate prediction, .5 indicative of strong prediction). For the second and third aims, separate linear regression analyses were conducted for each group to investigate: (1) the association between anxiety and objective and subjective sleep quality; and (2) the relationship between objective and subjective measures of sleep quality, respectively. For the second aim in each model, each individual sleep quality variable was the outcome, whereas the SCAS z score was the predictor. For the third aim in each model, each individual objective sleep quality variable was the outcome, whereas the PSQI global score was the predictor. The b values, significance level (P < .05), and coefficient of determination (R2 as a percentage) of each model were reported.
RESULTS
For the CFS/ME group, a total of 50 families were initially approached to participate in the study. Of these, 29 families either declined to be involved or were not able to be contacted to be involved in the project. Therefore, our study had a CFS/ME recruitment rate of 42% (21 participated/50 approached). Sample characteristics of the CFS/ME group are shown in Table 1. The proportion of males and females did not differ between groups (χ2(1) = 0.66, P = .42). However, given that age significantly differed between groups (t(164) = −2.54, P = .01), age was used as a covariate in the remaining regression analyses. There were also marked differences in sleep aids taken (ie, 81% of the CFS/ME sample were on melatonin, compared with 0% in the control sample), and school attendance. When comparing the groups of participants who completed the SCAS (n = 18 CFS/ME and n = 139 controls), 50% of CFS/ME participants (n = 9) were classified as being in the clinical range for total anxiety, compared with 23% in the healthy control participants (n = 32). Of participants who completed the PSQI (n = 16 CFS/ ME and n = 139 controls), 100% CFS/ME participants classified as “poor sleepers” (n = 16), compared with 58% of the healthy control participants (n = 80).
Table 1.
Sample characteristics of adolescent patients with chronic fatigue syndrome/myalgic encephalomyelitis.

The first aim was to investigate overall sleep quality in adolescents with CFS/ME compared with healthy adolescent controls. Mean and standard deviation (SD) for objective and subjective sleep data are shown in Table 2, along with the mean difference between groups. For the objective measures, as hypothesized, the CFS/ME adolescent group demonstrated significantly longer SOL, TIB, TST, and later RT, compared with controls (all P < .005). Upon inspection of β values, the greatest magnitude of difference was found for TIB and TST (β = .5). Nevertheless, the groups displayed similar levels of SE, BT, and WASO (all P > .05, β < .2). For the subjective measures, as expected, the CFS/ME adolescent group reported significantly poorer sleep quality than controls (P < .001, β = .5), with magnitude of the global PSQI scores in the patient group almost double that of the healthy adolescents (see Table 2).
Table 2.
Mean differences between patients with chronic fatigue syndrome/myalgic encephalomyelitis and controls on sleep quality and anxiety.
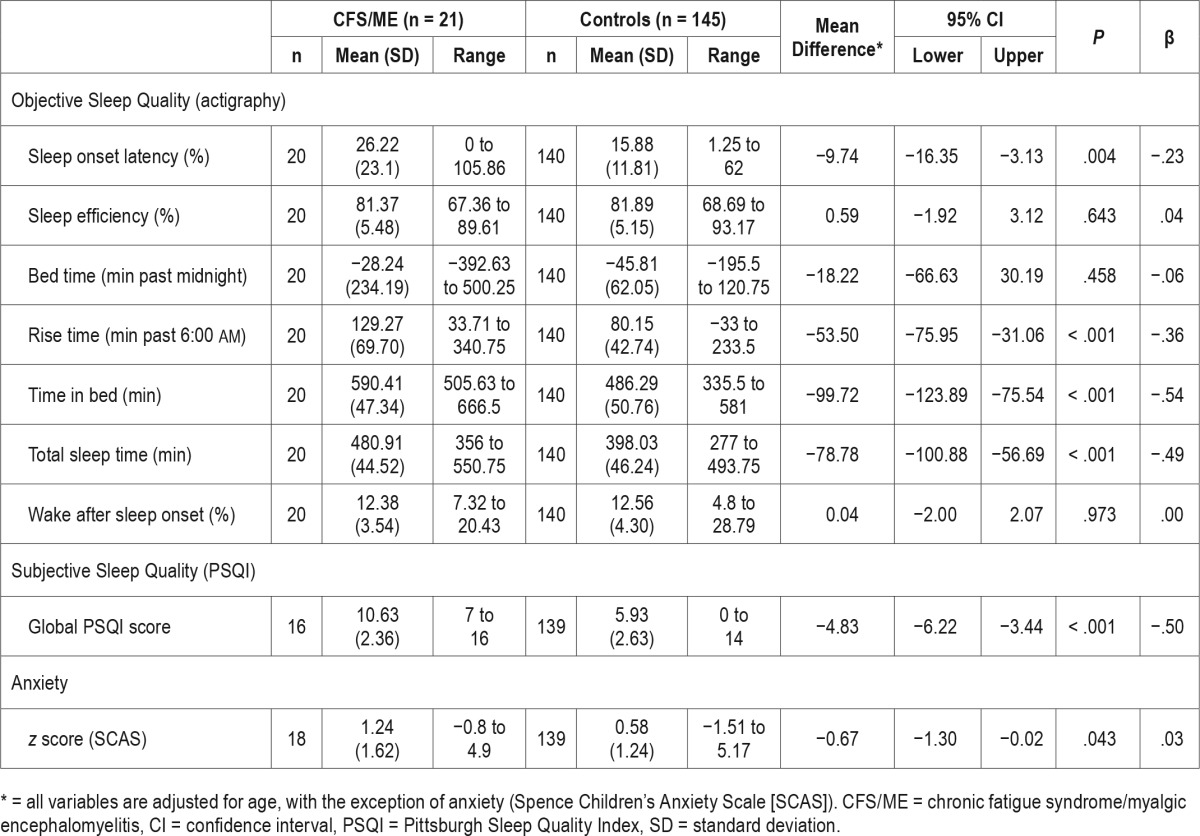
In terms of anxiety, adolescents with CFS/ME displayed significantly higher levels of anxiety on average than the control group, although this relationship was weak (P = .04, β < .2), with the CFS/ME group showing a higher mean z score on normative standards (z = 1.24) than the healthy control group (z = 0.58). All findings for the first aim held when re-run with sex as an additional covariate.
The second aim was to investigate the relationship between anxiety and objective and subjective sleep quality within each group. Mean and SD for the relevant variables are shown in Table 3, along with the regression results. For all variables, Fisher exact tests revealed that sex was not significantly different within each group (all P > .05), and was therefore not used as a covariate in the analysis. Age was also not used as a covariate given that the SCAS z scores were age-adjusted. Anxiety levels were not significantly associated with any of the objective sleep quality variables in either group, although anxiety levels were found to explain a substantial amount of variance in TST (R2 = 21%, β = −.5, P > .05) in the CFS/ME group. In contrast, a significant association was found with subjective sleep quality in both groups, where a higher level of anxiety was related to greater severity of self-reported sleep disturbance on the PSQI (CFS/ME group: b = 0.78; 95% confidence interval [CI]: 0.07, 1.48; P < .05; Control group: b = 0.79; 95% CI: 0.46, 1.13; P < .001). This relationship was stronger in the CFS/ME group (β = .6) compared with the control group (β = .4), with anxiety levels individually explaining approximately 32% of the variance in sleep disturbance in the CFS/ ME group, and 14% of the variance in the control group. All findings for the second aim held when re-run with sex, age, and a group × SCAS interaction term as additional covariates.
Table 3.
Association of anxiety (Spence Children's Anxiety Scale, z-score) with sleep quality within each group.
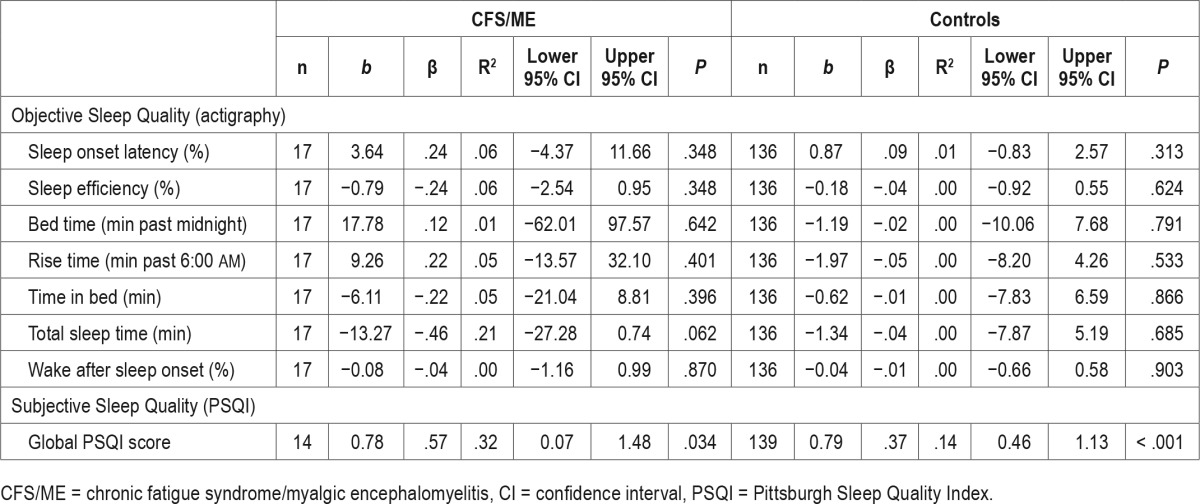
The third aim was to investigate the relationship between objective and subjective sleep measures. Self-reported sleep disturbance on the PSQI was not found to relate to any of the objective sleep measures, with the exception of SOL in the control group, where longer SOL was weakly related to greater severity of sleep disturbance in controls (b = 1.04; β = .2; 95% CI: 0.30, 1.80; P = .006).
Finally, other characteristics such as illness duration, school attendance, and time spent using electronic devices were not significant predictors of sleep quality (objective or subjective) (all P > .05), and as such, regression results were not shown.
DISCUSSION
This study was able to offer a unique and valuable perspective by providing objective and subjective evidence of sleep disturbance in adolescents with CFS/ME compared with healthy adolescent controls.
The first main finding was that, on average over a 2-week period, adolescents with CFS/ME were found to have significantly poorer sleep quality than healthy adolescent controls on a range of objective and subjective measures. On an objective basis, adolescents with CFS/ME demonstrated longer SOL, TIB, TST, and later RT, when their sleep was recorded via actigraphy watches. This may suggest that these patients have difficulty initiating sleep, and that they have a longer sleep time that proceeds later into the morning. Longer sleep latency in patients with CFS/ME compared with healthy controls has been demonstrated previously in adults,47,48 and in children and adolescents tested with actigraphy,18 and may be the result of variability in parasympathetic function at sleep onset (ie, nocturnal vagal modulation of heart rate).49 In contrast, the CFS/ ME patient group showed similar levels of objective SE to controls (ie, the CFS/ME group were spending the same proportion of TST relative to TIB as the control group), suggesting that they did not experience more frequent waking or spend a greater amount of time awake while they were in bed compared to controls. This is consistent with previous studies in adults showing similar SE between CFS/ME participants and healthy controls,50 and suggests that the disturbances may not occur in every aspect of sleep. Nevertheless, other studies have shown contrasting results (ie, similar TST and SOLs across CFS/ME and control groups,20,22 poorer SE in the CFS/ME patient group versus controls20). Importantly, these latter studies were not conducted using actigraphy, and only analyzed data over 1 night. It is possible that in comparison with techniques such as polysomnography, actigraphy is able to identify information that is more reliable and reflective of true sleep patterns because it is performed in a natural home environment and usually over a longer time period. The current study may therefore provide a more accurate representation and characterization of sleep quality in a pediatric CFS/ME population. Of course, it is recognized that not all information relevant to sleep quality may be captured via actigraphy (ie, measurement of sleep stages, arousal, and abnormal movements), and the inference of the onset and maintenance of sleep from the absence of movement recorded by the actigraphy watch may underestimate night wakings (and therefore SE) in participants that are lying still but are otherwise awake.
The use of different measures (ie, actigraphy, polysomnography) may partly account for the variability in results from previous studies investigating objective sleep quality in patients with CFS/ME, yet some other important factors must also be considered. For instance, the variability may also represent the heterogeneity of sleep phenotypes in CFS/ME. As suggested previously, CFS/ME may be characterized by a range of variable sleep features, rather than a unitary sleep profile.18 Attention must also be given to the specific CFS/ME sample used in the current study. Most of the CFS/ME sample used sleep aids to promote sleep quality, and although school attendance was shown not to play a significant role in influencing any of the objective sleep measures, the CFS/ME sample may have had less restrictions on their sleep routine to meet the early morning demands of school (ie, the CFS/ME group attended less than half of regular school days, compared with 100% school attendance in the control group). It is possible that the findings from the current study such as increased SOL, delayed RT, and increased TIB may reflect an altered wake/sleep/ work routine as a result of the illness, as well as heterogeneity in the CFS/ME population.
The second main finding was that, as hypothesized, the CFS/ ME adolescent group self-reported significantly poorer subjective sleep quality than their healthy peers on the PSQI. Consistent with previous reports of the adolescent CFS/ME population, unrefreshing sleep is a consistent feature of the condition,22,26–27 and was expected to be associated with the objective finding that patients with CFS/ME take longer to fall asleep. Indeed, differences or similarities between objective and subjective findings have seldom been assessed in the pediatric CFS/ME literature previously, and have the potential to be of clinical and diagnostic use. For instance, if self-report of unrefreshing sleep was correlated with objective signs of sleep disturbance, then simply asking patients about their subjective sleep in clinical interview may provide a quicker, more economically viable, and more feasible method of discovering sleep disturbance, instead of a thorough but expensive objective clinical sleep investigation. Alternatively, if objective and subjective sleep findings are found to be unrelated, different approaches to management of sleep disturbance may be required.
In the current study, our third main finding was that we did not observe a significant relationship between objective and subjective sleep quality. This is consistent with a previous study in adult CFS/ME where the PSQI was found not to correlate with objective measures such as sleep efficiency.50 While on the surface, this discrepancy between objective and subjective measures may appear to represent a “sleep quality misperception,” the possible reasons for this finding are complex and require further investigation. The one exception in the current study was that a significant correlation between objective and subjective measures was found in the control group where greater severity of subjective sleep disturbance was related to longer objective SOL. This suggested that healthy individuals taking longer to fall asleep had the perception that they had worse sleep quality overall, although it was unclear why this was the case only in the control group. It is possible that those with the self-perception of poorer sleep became more anxious about their sleep, and therefore took longer to fall asleep, but that our small CFS/ME sample was underpowered to detect this association in the patient group. Another potential reason for the lack of correlation between objective and subjective measures may be that objective sleep disturbance was underestimated via the actigraphy method (ie, night wakings may have been underrated in motionless but awake subjects), or that some of the neurophysiological disturbances associated with unrefreshing sleep in adolescent CFS/ME cannot be detected or captured by such measures as SE and SOL.23,50 Alternatively, it is possible that subjective findings of poor sleep quality may have been overestimated. Neu and colleagues50 suggest that anxiety may be an influencing factor in a response bias toward reporting subjective sleep disturbance in the CFS/ ME population, and this has previously been found in a pediatric CFS/ME sample.12 Subjective measures are inherently influenced by the observer's personal judgment, are prone to a large degree of variability and uncertainty,16 and may be influenced by other factors such as mood.50 Indeed, factors such as mood are supported in our CFS/ME sample, which demonstrated higher levels of anxiety than controls, whereas both groups showed a direct correlation between higher anxiety and greater subjective sleep disturbance. Nevertheless, the benefit of subjective measures is that they are a less expensive and often more feasible method of measuring sleep quality,25 and can reveal important information about the patient's sleep experience. Even in the absence of objective findings of sleep disturbance, subjective findings of poorer sleep quality have the potential to affect daily functional abilities, and therefore serve as a vital area of management for the clinician. Using objective and subjective sleep measures in conjunction, the current study was able to differentiate the patient's subjective experience of sleep from objective sleep findings and show that these may be distinct. This provides a potential avenue for treating anxiety in these adolescent populations, which may help their perception of sleep quality.
Finally, our study highlighted that a better understanding of the types of factors contributing to sleep disturbance is required. We found that illness-related variables such as illness duration, school attendance, and other factors such as time spent using an electronic device did not predict sleep quality in the CFS/ME group. However, it is possible that the size of our CFS/ME sample was underpowered to detect this influence. Further, it is acknowledged that our results may not be representative of all patients with CFS/ME because we had a study recruitment rate of 42% from our RCH CFS/ME Clinic, although recruitment rates of this nature are not uncommon in CFS/ME studies because of the nature of this illness (ie, participants may be unable to attend study appointments if bedridden). It is possible that patients with more severe difficulties were less likely to elect to participate in our study, potentially obscuring a relationship between sleep quality and factors such as illness duration and school attendance. Other factors may also contribute to sleep disturbance including pain, cognitive functioning, neuroendocrine responses, or dosage of prescription sleeping medications (ie, melatonin). It is also not yet clear to what extent sleep disturbance is contributing to other CFS/ ME symptoms such as pain and fatigue, which is commonly experienced by the CFS adolescent population.28 Although our study only investigated a limited number of factors, further research will need to expand on these other influencing variables, which may aid in more targeted interventions for this population. Our study was also limited in that a cross-sectional design was used, but further studies may determine whether sleep quality fluctuates across the course of the CFS/ME illness, and if so, how these disturbances may be treated.
To the authors' knowledge, this is the first study conducted on a pediatric CFS/ME sample to utilize both objective and validated subjective measures of sleep quality. Although it is possible that adolescents with CFS/ME who were not taking sleeping medication (ie, 81% of the CFS/ME sample were on melatonin) may show more significant or severe sleep disturbances, this study demonstrates that despite treatment with melatonin, adolescents with CFS/ME may continue to experience sleep disturbances and therefore other treatment avenues should be considered in future research. A strength of our study was that we were able to measure sleep quality over a 2-week period, which was more reflective of natural and typical sleep for these participants. In comparison, in polysomnography studies that only investigate 1 night's sleep, it is possible that first-night effects may influence the results in patients who do not have a primary sleep disorder.51 This may manifest as less TST and lower SE, due to the initial apprehension of being “under observation” or feeling uncomfortable with the multiple electrodes and cables used. In addition, sleep and wake times were not enforced in the current actigraphy study, further supporting the ecological validity of the data as typical of a normal night's sleep for adolescents.
Overall, these results suggest that adolescents with CFS/ME have significant sleep disturbances on a range of domains using an ecologically valid measure, and perceive their own sleep to be significantly disturbed compared with healthy adolescents. Furthermore, although psychological factors such as anxiety may not significantly contribute to objective sleep disturbance, it may play a significant role in influencing adolescents' perception of their quality of sleep. This research has provided us with a more comprehensive understanding of the complex relationship between CFS/ME and sleep, and suggests both objective and subjective factors may be important for clinicians to consider when treating adolescent patients with sleep disturbance.
DISCLOSURE STATEMENT
This study was funded by the Mason Foundation through the Australian and New Zealand Banking Group Limited Trustees and the Murdoch Childrens Research Institute. Research at the Murdoch Childrens Research Institute is supported by the Victorian Government's Operational Infrastructure Support Program. The authors have no conflicts of interest to declare.
ABBREVIATIONS
- BT
bed time
- CFS
chronic fatigue syndrome
- CI
confidence interval
- ME
myalgic encephalomyelitis
- PSQI
Pittsburgh Sleep Quality Index
- RCH
Royal Children's Hospital
- REM
rapid eye movement
- RT
rise time
- SCAS
Spence Children's Anxiety Scale
- SD
standard deviation
- SE
sleep efficiency
- SOL
sleep onset latency
- TIB
time in bed
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.Knight S, Harvey A, Towns S, et al. How is paediatric chronic fatigue syndrome/myalgic encephalomyelitis diagnosed and managed by paediatricians? An Australian Paediatric Research Network Study. J Paediatr Child Health. 2014;50(12):1000–1007. doi: 10.1111/jpc.12677. [DOI] [PubMed] [Google Scholar]
- 2.Nijhof SL, Maijer K, Bleijenberg G, Uiterwaal C, Kimpen J, van de Putte EM. Adolescent chronic fatigue syndrome: prevalence, incidence, and morbidity. Pediatrics. 2011;127(5):e1169–e1175. doi: 10.1542/peds.2010-1147. [DOI] [PubMed] [Google Scholar]
- 3.Blakemore SJ. The developing social brain: implications for education. Neuron. 2010;65(6):744–747. doi: 10.1016/j.neuron.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romeo RD. Adolescence: a central event in shaping stress reactivity. Dev Psychobiol. 2010;52(3):244–253. doi: 10.1002/dev.20437. [DOI] [PubMed] [Google Scholar]
- 5.Pinyerd B, Zipf WB. Puberty-timing is everything! J Pediatr Nurs. 2005;20(2):75–82. doi: 10.1016/j.pedn.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Telzer EH, Goldenberg D, Fuligni AJ, Lieberman MD, Galvan A. Sleep variability in adolescence is associated with altered brain development. Dev Cog Neurosci. 2015;14:16–22. doi: 10.1016/j.dcn.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand S, Kirov R. Sleep and its importance in adolescence and in common adolescent somatic and psychiatric conditions. Int J Gen Med. 2011;4:425–442. doi: 10.2147/IJGM.S11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor DJ, Jenni OG, Acebo C, Carskadon MA. Sleep tendency during extended wakefulness: insights into adolescent sleep regulation and behavior. J Sleep Res. 2005;14(3):239–244. doi: 10.1111/j.1365-2869.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- 9.Laberge L, Petit D, Simard C, Vitaro F, Tremblay RE, Montplaisir J. Development of sleep patterns in early adolescence. J Sleep Res. 2001;10(1):59–67. doi: 10.1046/j.1365-2869.2001.00242.x. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin Crabtree V, Williams NA. Normal sleep in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2009;18(4):799–811. doi: 10.1016/j.chc.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 11.O'Brien EM, Mindell JA. Sleep and risk-taking behavior in adolescents. Behav Sleep Med. 2005;3:113–133. doi: 10.1207/s15402010bsm0303_1. [DOI] [PubMed] [Google Scholar]
- 12.Snodgrass K, Harvey A, Scheinberg A, Knight S. Sleep disturbances in paediatric chronic fatigue syndrome: a review of current research. J Clin Sleep Med. 2015;11(7):757–764. doi: 10.5664/jcsm.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69(4):875–887. [PubMed] [Google Scholar]
- 14.Moore M, Kirchner HL, Drotar D, et al. Relationships among sleepiness, sleep time, and psychological functioning in adolescents. J Pediatr Psychol. 2009;34(10):1175–1183. doi: 10.1093/jpepsy/jsp039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spruyt K, Molfese DL, Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics. 2011;127(2):e345–e352. doi: 10.1542/peds.2010-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Zhao ZX. Objective and subjective measures for sleep disorders. Neurosci Bull. 2007;23(4):236–240. doi: 10.1007/s12264-007-0035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sadeh A. Commentary: comparing actigraphy and parental report as measures of children's sleep. J Pediatri Psychol. 2008;33(4):406–407. doi: 10.1093/jpepsy/jsn018. [DOI] [PubMed] [Google Scholar]
- 18.Ohinata J, Suzuki N, Araki A, Takahashi S, Fujieda K, Tanaka H. Actigraphic assessment of sleep disorders in children with chronic fatigue syndrome. Brain Dev. 2008;30(5):329–333. doi: 10.1016/j.braindev.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Kawabata M, Ueno T, Tomita J, et al. Temporal organization of rest defined by actigraphy data in healthy and childhood chronic fatigue syndrome children. BMC Psychiatry. 2013;13:281. doi: 10.1186/1471-244X-13-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stores G, Fry A, Crawford C. Sleep abnormalities demonstrated by home polysomnography in teenagers with chronic fatigue syndrome. J Psychosom Res. 1998;45(1):85–91. doi: 10.1016/s0022-3999(98)00024-5. [DOI] [PubMed] [Google Scholar]
- 21.Tomoda A, Jhodoi T, Miike T. Chronic fatigue syndrome and abnormal biological rhythms in school children. J Chron Fatigue Syndr. 2001;8(2):29–37. [Google Scholar]
- 22.Knook L, Kavelaars A, Sinnema G, Kuis W, Heijnen CJ. High nocturnal melatonin in adolescents with chronic fatigue syndrome. J Clini Endocrinol Metab. 2000;85(10):3690–3692. doi: 10.1210/jcem.85.10.6857. [DOI] [PubMed] [Google Scholar]
- 23.Jackson ML, Bruck D. Sleep abnormalities in chronic fatigue syndrome/ myalgic encephalomyelitis: a review. J Clin Sleep Med. 2012;8(6):719–728. doi: 10.5664/jcsm.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lunsford-Avery JR, LeBourgeois MK, Gupta T, Mittal VA. Actigraphic measured sleep disturbance predicts increased positive symptoms in adolescents at ultra high-risk for psychosis: a longitudinal study. Schizophr Res. 2015;164(1-3):15–20. doi: 10.1016/j.schres.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lycett K, Mensah F, Hiscock H, Sciberras E. Comparing subjective measures of behavioral sleep problems in children with ADHD: a cross-sectional study. Sleep Med. 2015;16(11):1377–1380. doi: 10.1016/j.sleep.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Bell DS. Diagnosis of chronic fatigue syndrome in children and adolescents: special considerations. J Chron Fatigue Syndr. 1995;1(3-4):29–36. [Google Scholar]
- 27.Knight S, Harvey A, Lubitz L, et al. Paediatric chronic fatigue syndrome: complex presentations and protracted time to diagnosis. J Paediatri Child Health. 2013;49(11):919–924. doi: 10.1111/jpc.12425. [DOI] [PubMed] [Google Scholar]
- 28.Le Bon O, Fischler B, Hoffmann G, et al. How significant are primary sleep disorders and sleepiness in the chronic fatigue syndrome? Sleep Res. 2000;3(2):43–48. [PubMed] [Google Scholar]
- 29.Stinson JN, Hayden JA, Ahola Kohut S, et al. Sleep problems and associated factors in children with juvenile idiopathic arthritis: a systematic review. Pediatr Rheumatol Online J. 2014;12:19. doi: 10.1186/1546-0096-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valrie CR, Bromberg MH, Palermo T, Schanberg LE. A systematic review of sleep in pediatric pain populations. J Dev Behav Pediatr. 2013;34(2):12–18. doi: 10.1097/DBP.0b013e31827d5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pillai V, Steenburg LA, Ciesla JA, Roth T, Drake CL. A seven day actigraphy-based study of rumination and sleep disturbance among young adults with depressive symptoms. J Psychosom Res. 2014;77(1):70–75. doi: 10.1016/j.jpsychores.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Jason LA, Bell DS, Rowe K, et al. A pediatric case definition for myalgic encephalomyelitis and chronic fatigue syndrome. J Chron Fatig Syndr. 2006;13(2-3):1–44. [Google Scholar]
- 33.Bei B, Allen N, Nicholas CL, Dudgeon P, Murray G, Trinder J. Actigraphy-based sleep during school and vacation periods: a naturalistic study of restricted and extended sleep opportunities in adolescents. J Sleep Res. 2014;23(1):107–117. doi: 10.1111/jsr.12080. [DOI] [PubMed] [Google Scholar]
- 34.Boyne K, Sherry DD, Gallagher PR, Olsen M, Brooks LJ. Accuracy of computer algorithms and the human eye in scoring actigraphy. Sleep Breath. 2013;17(1):411–417. doi: 10.1007/s11325-012-0709-z. [DOI] [PubMed] [Google Scholar]
- 35.Carskadon MA, Acebo C. Regulation of sleepiness in adolescents: update, insights, and speculation. Sleep. 2002;25(6):606–614. doi: 10.1093/sleep/25.6.606. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Liu L, Owens JA, Kaplan DL. Sleep patterns and sleep problems among school children in the United States and China. Pediatrics. 2005;115(1 Suppl):241–249. doi: 10.1542/peds.2004-0815F. [DOI] [PubMed] [Google Scholar]
- 37.Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6):602–612. doi: 10.1016/j.sleep.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Lacks P. Daily sleep diary. In: Herson M, Bellack AS, editors. Dictionary of Behavioural Assessment Techniques. New York, NY: Pergamon Press; 1988. pp. 162–164. [Google Scholar]
- 39.Libman E, Fichten CS, Bailes S, Amsel R. Sleep questionnaire versus sleep diary: which measure is better? Int J Rehab Health. 2000;5(3):205–290. [Google Scholar]
- 40.Chronic fatigue syndrome. Clinical practice guidelines — 2002. Med J Aust. 2002;176(>6 May Suppl):S17–S56. [PubMed] [Google Scholar]
- 41.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 42.Backhaus J, Junghanns K, Broocks A, RiemannI D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 43.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh sleep quality index. J Psychosom Res. 1998;45(1):5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 44.de la Vega R, Tome-Pires C, Sole E, et al. The Pittsburgh Sleep Quality Index: validity and factor structure in young people. Psychol Assess. 2015;27(4):e22–e27. doi: 10.1037/pas0000128. [DOI] [PubMed] [Google Scholar]
- 45.Spence SH. A measure of anxiety symptoms among children. Behav Res Ther. 1998;36(5):545–566. doi: 10.1016/s0005-7967(98)00034-5. [DOI] [PubMed] [Google Scholar]
- 46.Spence SH, Barrett PM, Turner CM. Psychometric properties of the Spence Children's Anxiety Scale with young adolescents. J Anxiety Disord. 2003;7(6):605–625. doi: 10.1016/s0887-6185(02)00236-0. [DOI] [PubMed] [Google Scholar]
- 47.Fischler B. Review of clinical and psychobiological dimensions of the chronic fatigue syndrome: differentiation from depression and contribution of sleep dysfunctions. Sleep Med Rev. 1999;3(2):131–146. doi: 10.1016/s1087-0792(99)90020-5. [DOI] [PubMed] [Google Scholar]
- 48.Whelton CL, Salit I, Moldofsky H. Sleep, Epstein-Barr virus infection, musculoskeletal pain, and depressive symptoms in chronic fatigue syndrome. J Rheumatol. 1992;19(6):939–943. [PubMed] [Google Scholar]
- 49.Moldofsky H. The significance, assessment, and management of nonrestorative sleep in fibromyalgia syndrome. CNS Spectr. 2008;13(3 Suppl 5):22–26. doi: 10.1017/s1092852900026808. [DOI] [PubMed] [Google Scholar]
- 50.Neu D, Mairesse O, Hoffman G, et al. Sleep quality perception in the chronic fatigue syndrome: correlation with sleep efficiency, affective symptoms and intensity of fatigue. Neuropsychobiology. 2007;56(1):40–46. doi: 10.1159/000110727. [DOI] [PubMed] [Google Scholar]
- 51.Le Bon O, Minner P, Van Moorsel C, et al. First-night effect in the chronic fatigue syndrome. Psychiatr Res. 2003;120(2):191–199. doi: 10.1016/s0165-1781(03)00185-9. [DOI] [PubMed] [Google Scholar]


