Figure 6.
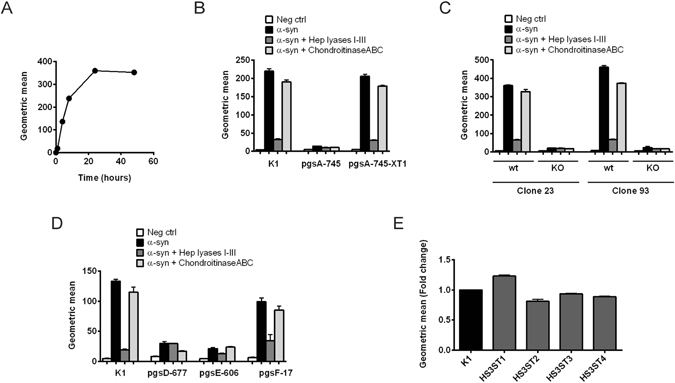
Internalization of α-synuclein fibrils by CHO cells deficient in different enzymes involved in GAG synthesis. pHrodo-α-synuclein fibrils were added to the cell media and their cellular internalization was analyzed with flow cytometry. Each cell line was treated with a mix of heparin lyases I, II and III or chondroitinase ABC (5 mU/ml) as a means to check the accuracy of the results obtained from the different lines. (A) Time course of pHrodo-α-synuclein fibrils internalization in CHO K1 (wt) cells. (B–E) Internalization of pHrodo-α-synuclein fibrils after incubation for 8 hr in CHO cells with different mutations. (B) Internalization of pHrodo-α-synuclein fibrils in CHO cells deficient in all GAGs (pgsA-745 strain), caused by insufficient xylosyltransferase activity, and in CHO pgsA-745 cells stably transfected with xylosyltransferase 1 (pgsA-745-XT1). (C) Internalization of pHrodo-α-synuclein fibrils in two different clones (clone 23 and 93) selected from CHO K1 cells, in comparison to the same clones where XylT2 has been knocked out. (D) Internalization of pHrodo-α-synuclein fibrils in CHO cells deficient in enzymes involved in heparan sulfate synthesis. The pgsD-677 strain lacks HS, due to deficiency in Ext1, which is required for polymerization of the heparan sulfate chain. pgsE-606 cells are deficient in N-sulfation of heparan sulfate chains and also show a lower general degree of heparan sulfate sulfation. pgsF-17 cells are deficient in 2-O-sulfation of heparan sulfate chains, but show relatively unchanged overall sulfation of heparan sulfate. (E) Internalization of pHrodo-α-synuclein fibrils in CHO K1 cells stably transduced with HS3ST1-4.
