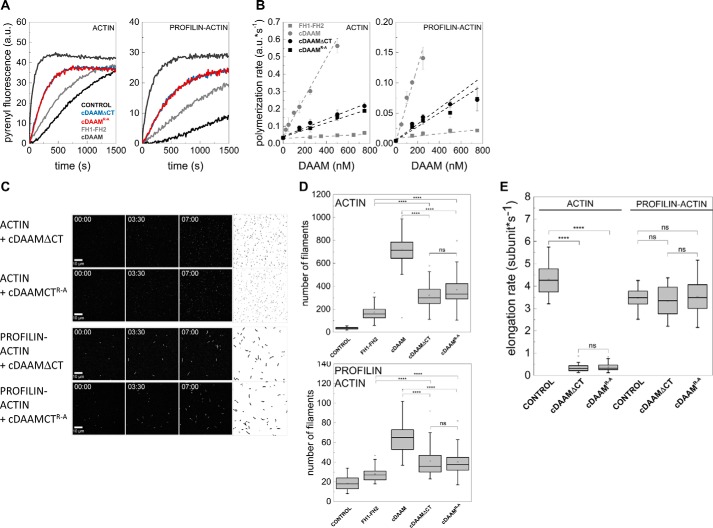
Figure 4.
Effects of DAAM DAD and CT regions in the presence of the native FH2 domain. A, representative pyrenyl traces of spontaneous and cDAAMArg–Ala- or cDAAMΔCT-catalyzed assembly of free G-actin and profilin/G-actin, as indicated. The data for FH1–FH2 and cDAAM from Fig. 2E are shown here as controls. Final concentrations are as follows: [actin] = 2 μm; [profilin] = 6 μm; [cDAAMArg–Ala] = 200 nm; [cDAAMΔCT] = 200 nm. B, cDAAMArg–Ala or cDAAMΔCT concentration dependence of the relative polymerization rate of free G-actin and profilin/G-actin, as indicated. Error bars, standard deviations, n = 3–4. Data obtained for FH1–FH2 and cDAAM from Fig. 2B are shown. C, TIRFM montages of actin assembly and representative skeletonized images showing the field of view of actin assembly in the absence and presence of cDAAMArg–Ala or cDAAMΔCT, as indicated (for spontaneous actin assembly see Fig. 2D). Scale bar, 10 μm, time = min/s. Final concentrations are as follows: [actin] = 0.5 μm; [profilin] = 2 μm; [cDAAMArg–Ala] = 100 nm; [cDAAMΔCT] = 100 nm. D, number of actin filaments nucleated spontaneously or in the presence of cDAAMArg–Ala or cDAAMΔCT derived from skeletonized images. Final concentrations as in C, n = 20–54. E, elongation rate of individual actin filaments polymerized from free and profilin/G-actin in the absence and presence of cDAAMArg–Ala or cDAAMΔCT. Final concentrations as in C, n = 39–79. a.u., arbitrary units; ns, not significant.