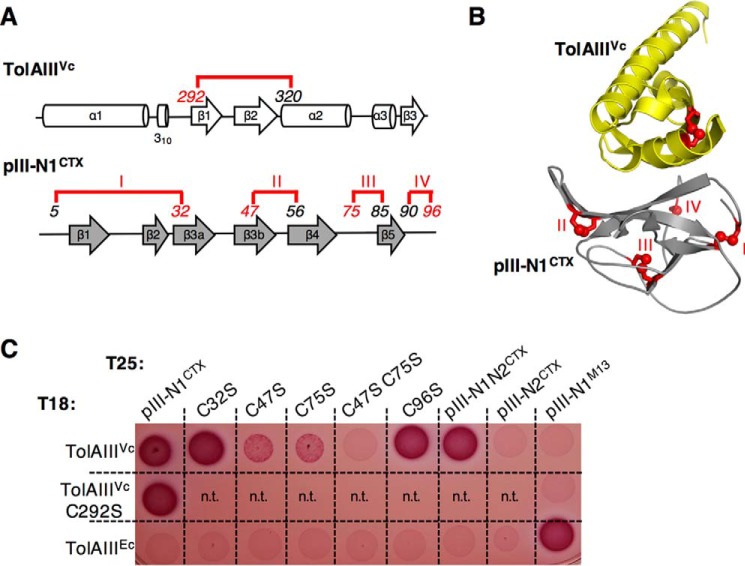
Figure 2.
pIII-N1CTX disulfide bonds II and III are required for TolAIIIVc interaction. A, schematic representation of disulfide bond localization (15) on the secondary structure of TolAIIIVc and pIII-N1CTX; B, representation of disulfide bonds on the crystal structure of the complex. Disulfide bonds in pIII-N1 are numbered I–IV and colored in red. Italic numbers refer to cysteine residue positions. C, oxidative bacterial two-hybrid assay: Oxi-BTH reporter cells producing the T25 domain fused to pIII-N1CTX, pIII-N1N2CTX, or pIII-N2CTX domains and the T18 domain fused to V. cholerae TolAIII were spotted on MacConkey plates. Variants bearing substitutions aimed to abolish each of the four disulfide bonds in the pIII-N1CTX domain (C32S, C47S, C75S, and C96S) or in TolAIIIVc (C292S) are presented. TolAIIIEc and pIII-N1M13 are used as a controls. n.t., not tested.