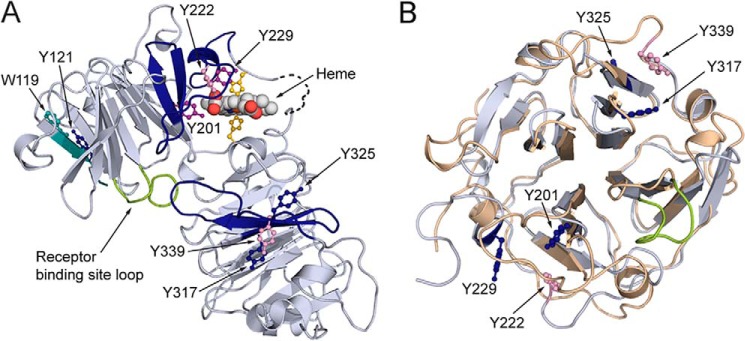
Figure 5.
Distribution of covalently oxidatively modified amino acids after hemopexin is exposed to RNS and ROS. The distribution of covalently and oxidatively modified amino acids after rabbit hemopexin is exposed to RNS and ROS. A, solely endogenously nitrated amino acids are in dark blue; those modified by ROS (treated with HOCl or tert-butyl hydroperoxide, see Table 2) are in pink, and those modified by both nitration and ROS are in purple. Notably, tyrosines 201, 222, and 229 reside in the heme-binding site consistent with the impaired heme binding and are clearly vulnerable to oxidative modification unless protected by heme binding. Also identified were tryptophan 119 and tyrosine 121 in the N-domain and tyrosines 317, 325, and 339 in the C-domains (see Tables 2 and 3). The peptides in which the three modified tyrosines in the N- and C-domains reside are contiguous; however, they are in different blades of the two homologous β-propellers. The relative conformation of the two domains in the open apoprotein structure is unknown. Certain modified residues are no longer solvent-accessible when heme is bound to hemopexin (shown in Fig. 3, E and F), although the receptor-binding site is. B, superimposing the N-domain's (wheat) and the C-domain's (blue/gray) homologous four-bladed β-propellers of hemopexin in the N to C orientation, the three oxidatively modified tyrosine residues in the heme-binding site reside on blade 4, whereas the three on the C-domain reside on blade 6, numbering the blades sequentially from the N to C terminus of hemopexin.