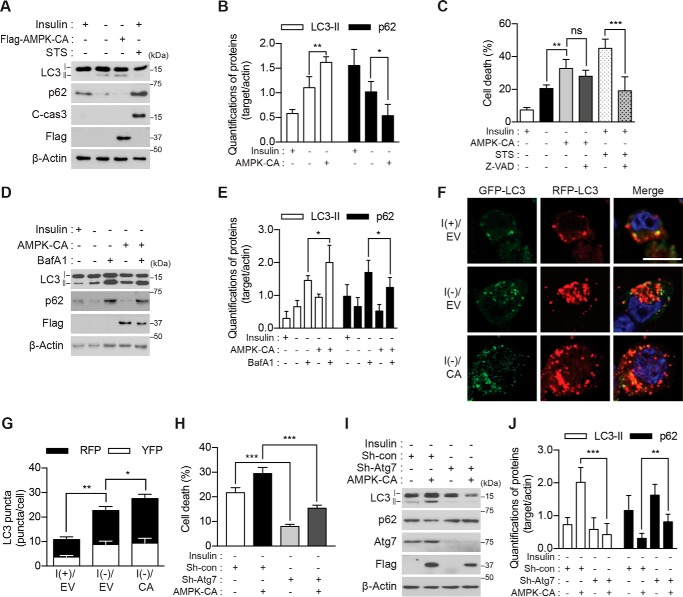
Figure 2.
Genetic activation of AMPK enhances ACD in I(−) HCN cells. A, overexpression of AMPK-CA increased the level of LC3-II and decreased that of p62 but did not activate caspase-3 following insulin withdrawal for 5 h. STS (0.5 μm for 8 h) was used as a positive control for apoptosis. C-cas3, cleaved caspase-3. B, quantification of LC3-II and p62 after normalization to β-actin (n = 4). C, AMPK-CA increased cell death more than did I(−) alone. Z-VAD (20 μm) failed to reduce cell death induced by AMPK-CA but efficiently reduced STS-induced apoptosis (n = 5). D, AMPK-CA increased autophagic flux in I(−) HCN cells. Insulin was withdrawn for 5 h, and BafA1 (20 nm) was added 1 h before sampling. E, quantification of LC3-II and p62 after normalization to β-actin (n = 5). F, AMPK-CA (CA) increased autophagic flux. Autophagic flux was measured using tandem mRFP-GFP-LC3 after 5-h insulin withdrawal. G, analysis of F. The red and yellow puncta were counted from three independent experiments (n = 29). H, Atg7 knockdown abrogated AMPK-CA-triggered cell death. Cell death was measured 24 h after insulin withdrawal (n = 6). I, Atg7 knockdown abrogated AMPK-CA-triggered autophagic flux. AMPK-CA failed to affect LC3-II or p62 in Sh-Atg7 HCN cells analyzed 5 h after insulin withdrawal. J, quantification of LC3-II and p62 after normalization to β-actin (n = 9). Error bars represent ±S.D. from independent assays. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ns, not significant. Scale bar, 10 μm. EV, empty vector; con, control.