Figure 5.
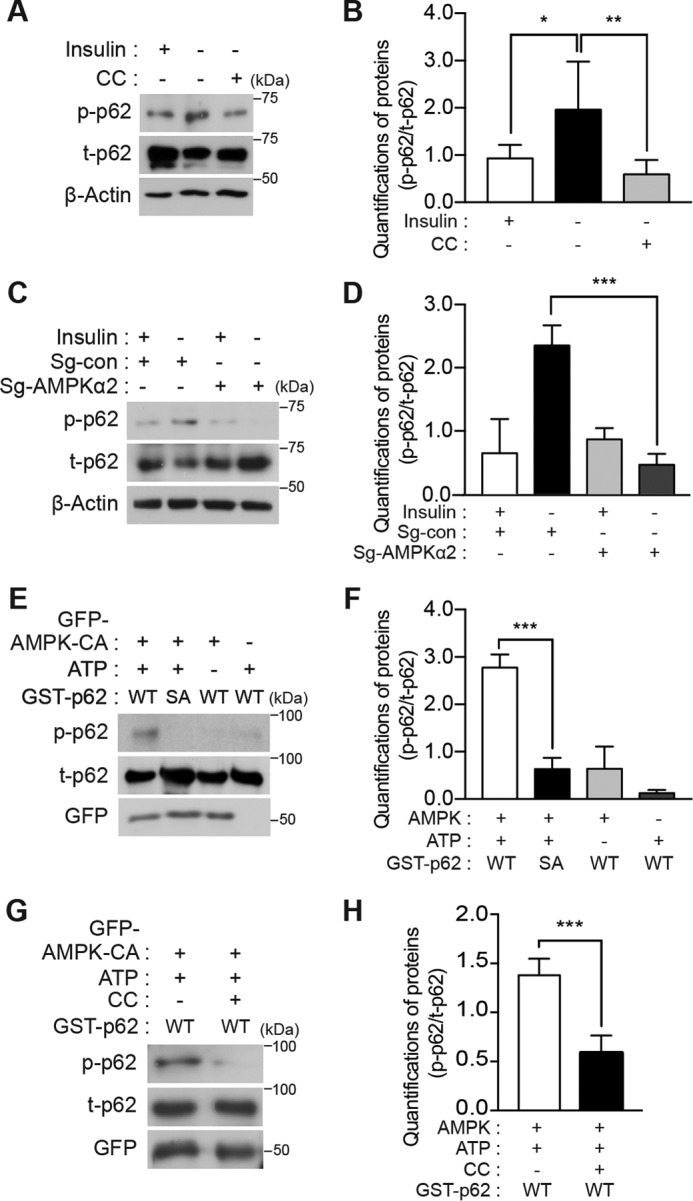
Serine phosphorylation at a novel site in p62 is mediated by AMPK. A, endogenous p62 was phosphorylated in an AMPK-dependent manner in HCN cells. Insulin withdrawal for 5 h induced phosphorylation of p62 Ser-293, which was detected by using a phosphospecific antibody. CC (0.5 μm) reduced Ser-293 phosphorylation. B, quantification of p62 Ser-293 phosphorylation (p-p62) after normalization to total p62 (t-p62) following CC treatment (n = 6). C, AMPK α2 KO reduced Ser-293 phosphorylation. D, quantification of p62 Ser-293 phosphorylation (p-p62) after normalization to total p62 (t-p62) in AMPK α2 KO HCN cells (n = 6). E, hp62-WT, but not hp62-S294A mutant (SA), was phosphorylated in vitro by the enriched AMPK immune complex. Phosphorylation was detected by Western blotting with phosphospecific antibody. F, quantification of in vitro kinase assay results (n = 4). G, treatment of the AMPK immune complex with CC (1 μm) for 1 h abolished p62 phosphorylation. H, quantification of in vitro kinase assay results with CC (n = 3). Error bars represent ±S.D. from independent assays. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
