Figure 1.
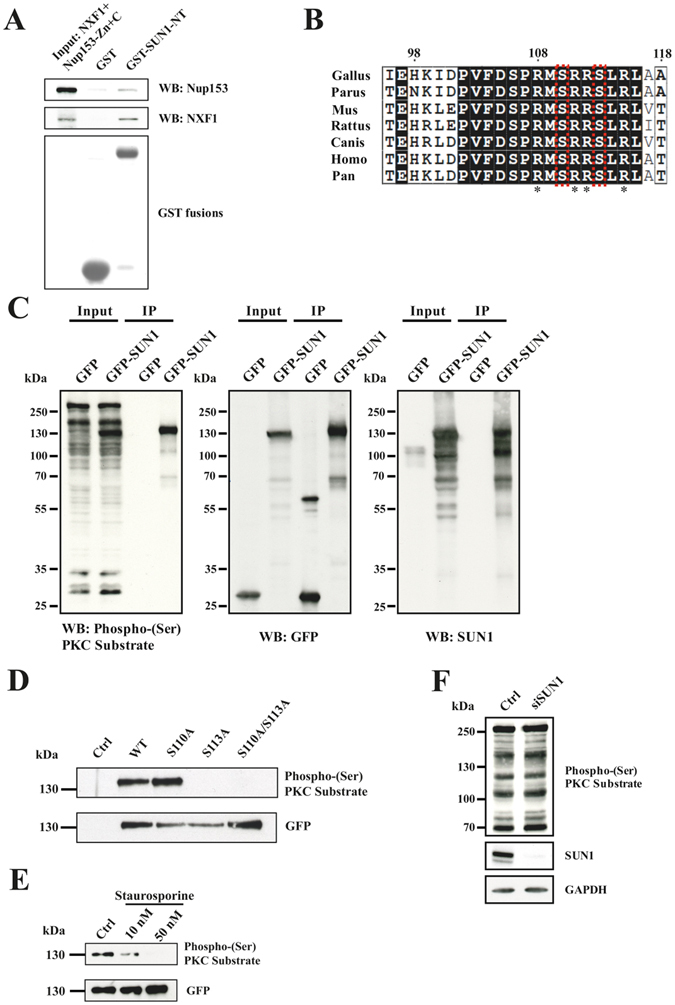
SUN1 interacts differentially with NUP153 and NXF1 and is a substrate of protein kinase C. (A) Competition between NXF1 and NUP153 for SUN1 binding. Recombinant NXF1/NXT and Nup153-Zn+C released from GST (input) were used for pull down experiments with GST (control) and GST-SUN1-NT. NXF1 and NUP153 were detected by antibodies. Below the pull down a western blot detecting the GST proteins is shown. (B) Comparison of the highly conserved predicted PKC phosphorylation site in SUN proteins from various species. The numbering corresponds to human SUN1 (ACCESSION: EAL23707). The S residues S110 and S113 are boxed in red. Stars point out the consensus sequence. Boxed in black, identical amino acid residues, boxed in white, similar amino acid residues. (C) Cell lysates from GFP and GFP-SUN1 expressing HeLa cells (Input) were used for pull downs using GFP trap beads (IP) and probed with Phospho-(Ser) PKC Substrate, GFP and SUN1 antibodies as indicated. (D) WT SUN1 and SUN1 proteins carrying point mutations at the indicated positions were expressed as GFP fusions in HeLa cells and precipitated using GFP trap beads. The blot was probed with Phospho-(Ser) PKC Substrate and GFP antibodies. For control, cells expressing GFP were used. (E) Staurosporine treatment reduces SUN1 phosphorylation. Staurosporine was applied for 6 hours at the indicated concentrations. The immunoprecipitated material was probed with Phospho-(Ser) PKC Substrate followed by GFP antibodies. (F) SUN1 knockdown does not affect the staining pattern of Phospho-(Ser) PKC Substrate antibodies in whole cell lysates. siRNA mediated SUN1 knockdown was confirmed by labelling with SUN1 antibodies. GAPDH was used as loading control.
