Abstract
This article presented an innovative data of feasibility to produce Arachidonic acid (ARA), as added-value Polyunsaturated Fatty Acids (PUFA), among other lipids from Mortierella elongata, using simulated low cost sugarcane wastewater, vinasse, as a carbon source. Data from lipids quantification by total lipids extraction and by lipid classes was presented. M. elongata was able to produce 156.45mg of ARA per g of total lipids.
Keywords: Arachidonic acid (ARA), Lipids, Mortierella elongata, Polyunsaturated Fatty Acids (PUFA), Sugarcane wastewater- vinasse
Specifications Table
| Subject area | Biology |
| More specific subject area | Lipids production by biotechnological process and their extraction |
| Type of data | Table, text file, graph, figure |
| How data was acquired | Gas Chromatography - Flame Ionization Detector (GC-FID) |
| Data format | Raw data collection and analysis |
| Experimental factors | Biomass production and oven drying and lipids extraction, classification, transesterification and analyzed in the GC-FID. |
| Experimental features | The microorganism was cultivated in low cost simulated sugarcane wastewater, vinasse. |
| Data source location | Mortierella elongata (CBS 121.71) was acquired from the CBS-KNAW Fungal Biodiversity Centre, Netherlands. All experiments were developed in Campinas-SP, Brazil. |
| Data accessibility | Data are presented in this article |
Value of the data
-
•
The obtained data are innovative and important in the research area of biotechnology providing results of lipids production by M. elongata, a fungus, using low cost substrate, sugarcane wastewater vinasse, applied in Nutrition and Pharmaceutical Industry.
-
•
Sugarcane vinasse, the main residue of ethanol process production, is a promising low cost carbon source and produced in large amounts. Since there is estimative of a growth in Brazilian domestic ethanol consumption of 58.8 billion liters by 2019 [1], with a corresponding vinasse production of 588 billion liters.
-
•
The data can be most useful for the researcher, research student, academician and industry to acquire innovative process development and production.
-
•
The described data identify a sustainable mechanism and with low cost to produce added-value lipid mainly, Arachidonic acid, since it is recommend injection of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and Arachidonic acid (ARA) in the infant [2], and for adults prevent coronary heart disease [3] and cancer [4].
1. Data
Mortierella elongata is an oleaginous fungus able to accumulate lipids. The data of lipids extraction from M. elongata are presented in the Table 1. The total lipids extraction from M. elongata were 14.95% and 8.54%, by MC and HIP method, respectively, using simulated low cost sugarcane wastewater, vinasse as a carbon source. Triacylglycerol (TGA) was highest lipid class observed, which is in accordance with previous studies for genus Mortierella, using other substrate [5]. Considering the HIP and MC lipids extraction process, the mixture Hexane/Isopropanol has less polar lipid interaction properties than Chloroform/Methanol [6], which reduce the extraction of phospholipids as observed in the data presented in the Table 1.
Table 1.
Lipids classes in percentage extracted from Mortierella elongata.
| Average of Lipids (%) (w:w) |
Method |
|
|---|---|---|
| MC | HIP | |
| TAG (% of TL) | 71.87% | 92.54% |
| NEFA (% of TL) | 25.74% | 4.68% |
| PP (% o TL) | 2.43% | 2.78% |
| TOTAL LIPIDS (% of DW) | 14.95% | 8.57% |
TAG – triacylglycerol, NEFA – free fatty acids, PP –phospholipids, TL – total lipids, DW – dry weight.
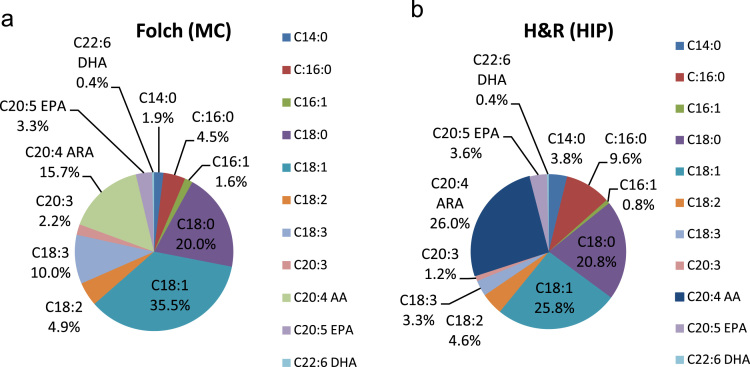
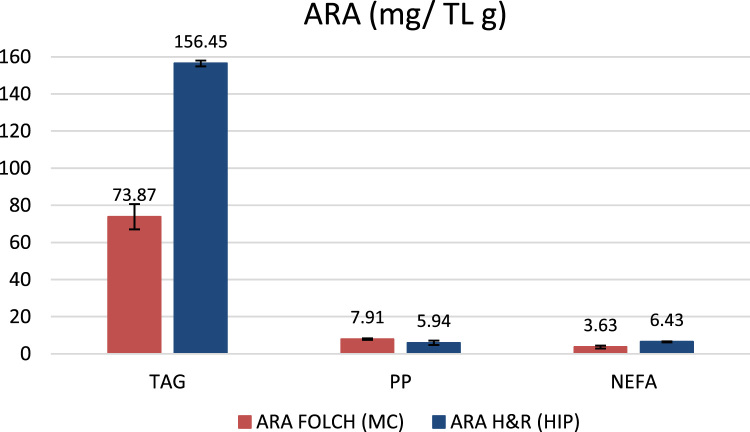
According to Fig. 1 (Chromatograms in the Supplementary data), it is presented the data profile of lipids by chain length and saturation, since C18:0, C18:1 and C20:4, the Arachidonic acid (ARA) were the predominant lipids. The Fig. 2 shows data of percentage and amount of ARA extracted by lipid class. In HIP method there was a high ARA extraction by percentage (26.0 %) of total fatty acids and in recovery weight (156.45mg of ARA g-1 of TGA and 168.81mg of ARA g-1 of TL) in five days of culture (Fig. 2), using simulated vinasse as a carbon source.
Fig. 1.
Main fatty acids profile of Mortiella elongata by a) Folch and b) Hara and Radin lipid extraction methods.
Fig. 2.
Arachidonic acid (ARA) concentration by lipids extraction method.
2. Experimental design, materials and methods
2.1. Microorganism cultivation
Mortierella elongata (CBS 121.71) was inoculated per 5 days, at 29 °C, in shaker at 200rpm, with culture medium simulating the main carbon sources of sugarcane vinasse [7], main sub-product of ethanol process production.
2.2. Simulated vinasse composition
Simulated sugarcane vinasse composition, based on the real wastewater characterization: (g/L): sucrose 10, glucose 6, glycerol 6, lactic acid 3, acetic acid 1, Yeast Extract 1, and 10% (v:v) of trace elements stock solution – (g/L): KH2PO4 0.65; (NH4)2SO4 14.00; MgSO4.7H2O 3.00; CaCl2.2H2O 39.97; FeSO4. 7H2O 0.05; ZnSO4.7H2O 0.013; MnSO4.H2O 0.016; CoCl2 0.02; NaNO3 28.00;KOH 20.00.
2.3. Lipids extraction
The oven dried biomass of Mortierella elongata (Dry Weight- DW) (80°C) was macerated and disrupted using HCl 1%, and the total lipids was extracted by Folch et al. [8] method, using Chloroform/Methanol (2:1) (CM) as solvents, and by Hara and Radin [9] method, using Hexane/Isopropanol (2:3) (HIP) as solvents. The total lipid (TL) was stratified by classes (phospholipids – PP, triacylglycerol - TAG, non-esterified fatty acids or free fatty acids – NEFA) using Solid-phase extraction (SPE) protocol [10].
2.4. Lipids quantification
The stratified total lipids and by class were transesterified with NaOH-methanol, Boron Trifluoride, and Hexane. The Fatty Acids Methyl Ester (FAME) were analyzed in the GC-FID (Chrompack 9001) using CP-Sil 88 capillary column (Chromatograms in the Supplementary data).
Acknowledgements
The authors are grateful to São Paulo Research Foundation (FAPESP) for funding this Project (2014/07848-8).
Footnotes
Transparency data associated with this article can be found in the online version at doi:10.1016/j.dib.2017.07.015.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dib.2017.07.015.
Contributor Information
Bruna Soares Fernandes, Email: brunasofer@hotmail.com.
José Geraldo da Cruz Pradella, Email: jpradella@bioetanol.org.br.
Transparency document. . Supporting information
Supplementary material
Appendix A. . Supplementary material
Supplementary material
References
- 1.MAPA. Ministério da Agricultura, Pecuária e Abastecimento. 〈http://www.agricultura.gov.br/〉, 2009. (Accessed 01 May 2009).
- 2.FAO, F. and agriculture organization of the united nations. Fats and Fatty Acids in Human Nutrition Report of an Expert Consultation, Food and Agriculture Organization of the United Nations, Rome, 2010. [PubMed]
- 3.S. Cunnane, C.A. Drevon, B. Harris, A. Sinclair, A. Spector. International Society for the Study of Fatty Acids and Lipids, 2004, pp. 1–22.
- 4.Meng H., Liu Y., Lai L. Diverse ways of perturbing the human arachidonic acid metabolic network to control inflammation. Acc. Chem. Res. 2015;48:2242–2250. doi: 10.1021/acs.accounts.5b00226. [DOI] [PubMed] [Google Scholar]
- 5.Wang L., Chen W., Feng Y., Ren Y., Gu Z., Chen H., Wang H., Thomas M.I., Zhang B., Berquin I.M., Li Y., Wu J., Zhang H., Song Y., Liu X., Norris J.S., Wang S., Du P., Shen J., Wang N., Yang Y., Wang W., Feng L., Ratledge C., Zhang H., Chen Y.Q. Genome characterization of the oleaginous fungus mortierella alpina. PLoS One. 2011;6:e28319. doi: 10.1371/journal.pone.0028319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halim R., Danquah M.K., Webley P.A. Extraction of oil from microalgae for biodiesel production a review. Biotechnol. Adv. 2012;30:709–732. doi: 10.1016/j.biotechadv.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Doelsch E., Masion A., Cazevieille P., Condom N. Spectroscopic characterization of organic matter of a soil and vinasse mixture during aerobic or anaerobic incubation. Waste Manag. 2009;29:1929–1935. doi: 10.1016/j.wasman.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Folch J., Lees M., Stanley G.H. Sloane. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 9.Hara A., Radin N.S. Lipid extraction of tissues with a low-toxicity solvent. Anal. Biochem. 1978;90:420–426. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- 10.Burdge G.C., Wright P., Jones A.E., Wootton S.A. A method for separation of phosphatidylcholine, triacylglycerol, non-esterified fatty acids and cholesterol esters from plasma by solid-phase extraction. Br. J. Nutr. 2000;84:781–787. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material