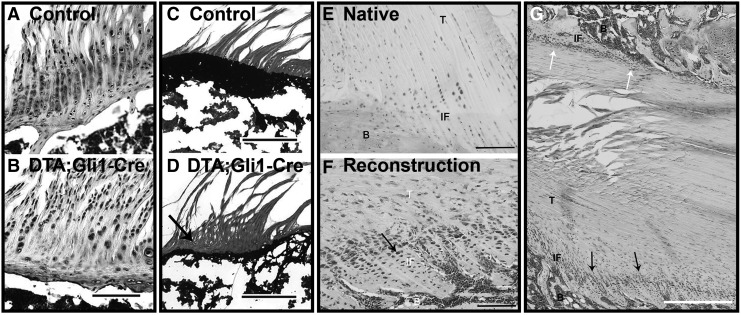
FIG. 2.
The role of hedgehog signaling in enthesis formation and healing. Parallel columns of fibrochondrocytes are embedded in a proteoglycan-rich extracellular matrix (A) that has undergone robust mineralization (C) by postnatal day 42 in the developing rotator cuff enthesis of a mouse, as shown through Safranin O (A, B) and Von Kossa (C, D) staining, respectively. Conditional ablation of Hh-responsive cells disrupts enthesis formation with reductions in proteoglycan (B) and mineral content (D, black arrow). Adapted with permission from Schwartz et al.202 Cells located at the native ACL–bone interface in a mature rat do not strongly express Hh mediators (E). Conversely, Hh signaling is strongly upregulated in cells at the tendon graft–bone tunnel interface following ACL reconstruction (F). Focal stresses appear to differentially affect Hh signaling and tissue organization (G), as interfaces presumably under greater tension (bottom, black arrows) exhibit greater organization and fibrochondrocyte alignment than unloaded interfaces (G, white arrows). ACL, anterior cruciate ligament; B, bone; Hh, hedgehog; IF, interface; T, tendon. Adapted with permission from Carbone et al.203