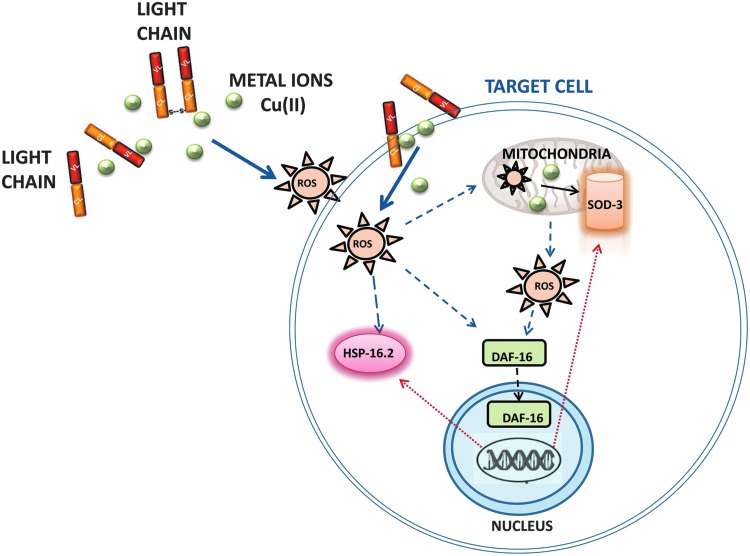
FIG. 8.
Proposed model for metal ion involvement in the mechanism underlying the LC-induced toxicity. Based on our current knowledge, redox-active transition metals, particularly copper, drive the ability of cardiotoxic LC to produce ROS in vivo. The excessive production of ROS can directly target the pharyngeal cells, damaging the organelle functions and the ultrastructure, particularly at the mitochondrial level. ROS can be also produced as a result of the mitochondrial dysfunction requiring copper and iron for the activation of the enzymes involved in the oxidative phosphorylation pathway. Intracellular signaling events aimed at limiting and repairing the stress-induced damage are activated. Mitochondria reacts to ROS by inducing the expression of the scavenger protein SOD-3. In addition, the chaperone HSP-16.2, an αB-crystallin-related protein, is activated as well as the nuclear translocation of the FOXO/DAF-16 transcription factor. This last one can trigger a secondary ROS-induced cellular response by inducing the transcription of stress-responsive genes, including hsp-16.2 and sod-3, and controlling longevity. FOXO, forkhead transcription factors.