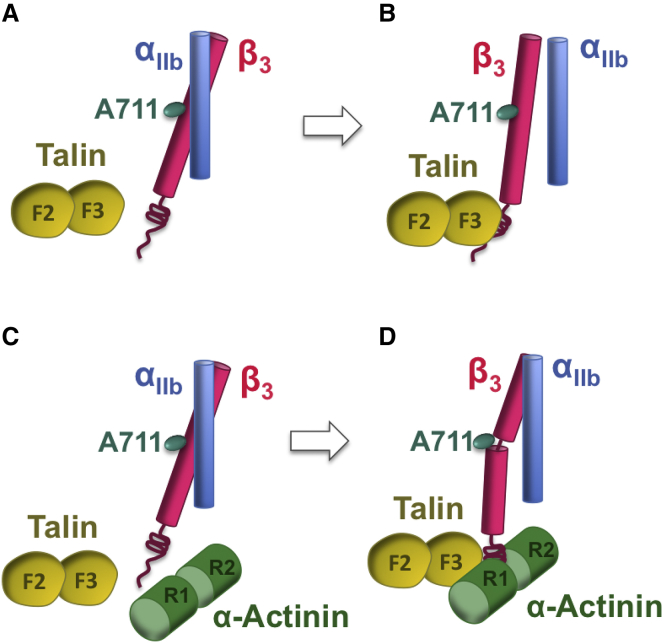
Figure 7.
Possible molecular mechanism by which α-actinin suppresses integrin-mediated signaling. (A) In the inactive conformation of integrin, the TMD domains of integrin subunits directly interact. (B) As talin associates with the cytoplasmic tail of β3-integrin, the IMC interaction between integrin subunits is broken, changing the distance between αIIb and β3 subunits without altering the TMD conformation. (C) Talin and α-actinin compete for binding to the β3-tail. For clarity, only R1 and R2 repeats of α-actinin are shown. (D) α-Actinin directly engages with both the β3-tail and talin head and prevents talin from binding to integrin. It also induces a kink at A711 in the β3-TMD, which most likely impairs integrin signaling. For clarity, the full-length α-actinin is not shown but it was included in all simulations. To see this figure in color, go online.