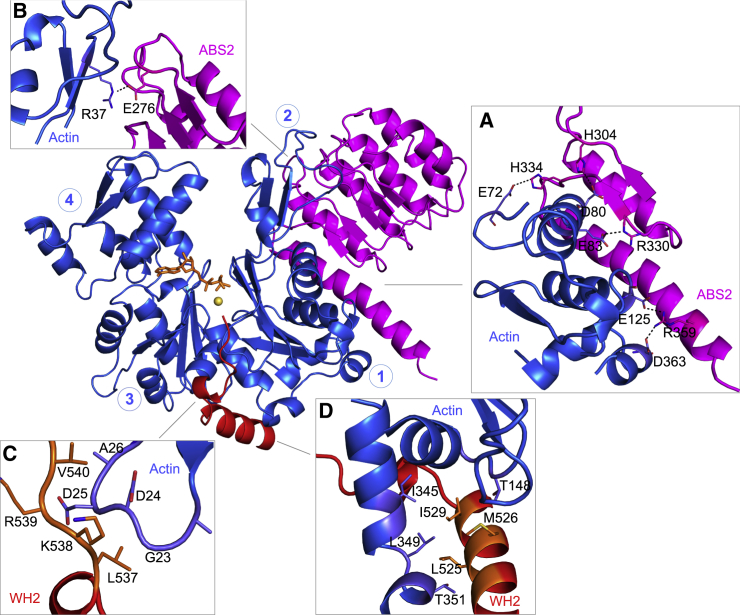
Figure 3.
Interactions between actin and Lmod2. Residues involved in interactions are labeled and their side chains are shown for actin and Lmod2’s ABS2 (A and B) and the WH2 domain (C and D). (A) Shown here are salt bridges between actin subdomain 1 and Lmod2 ABS2. (B) R37 in actin subdomain 2 interacts with E276 in Lmod2 ABS2. (C) The 537LKRV540 motif of the WH2 domain makes backbone interactions with a loop in actin subdomain 1, and V540 inserts into a hydrophobic pocket in actin. (D) The helix of the WH2 domain makes extensive hydrophobic contacts in the cleft formed between actin subdomains 1 and 3. To see this figure in color, go online.