Figure 4.
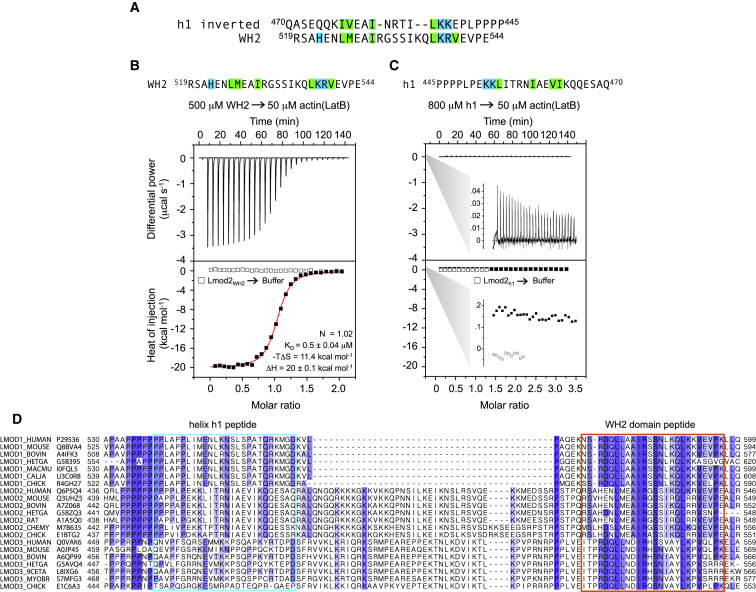
The WH2 domain binds actin whereas helix h1 does not. (A) Shown here is the alignment of the WH2 domain and the inverted helix h1 of Lmod2 proposed by Chen et al. (8) to explain their reported binding of these two sequences to the same site on the actin monomer. The canonical residues of the WH2 domain implicated in interactions with actin and their proposed equivalents in the inverted helix h1 are background-highlighted according to their chemical character. (B and C) Shown here are ITC titrations of the WH2 domain and helix h1 into LatB-actin. The titration of helix h1 is shown using the same scale as in (B), with zooms showing that the heat changes are ∼100-fold weaker for helix h1 than for the WH2 domain. The experiments were performed at 25°C and at the indicated protein concentrations. Open symbols correspond to control titrations of peptides into buffer. Only the titration of the WH2 domain could be fitted to a one-site binding isotherm (solid curve, parameters of the fit shown inside the graph), whereas helix h1 did not bind. Errors correspond to the SD of the fits. (D) Shown here is an alignment of a representative group of Lmod sequences for the region of the C-terminal extension encompassing from the PRD to the WH2 domain. The name of each sequence reflects the specific isoform, organism, and UniProt accession code. For each column, the amino acid conservation decreases from a dark to white background. The boxed regions span the length of the synthetic peptides used in the ITC experiments for the proposed helix h1 actin-binding site and the WH2 domain. Note that whereas the WH2 domain is highly conserved among Lmod isoforms and across different species, helix h1 is not. To see this figure in color, go online.