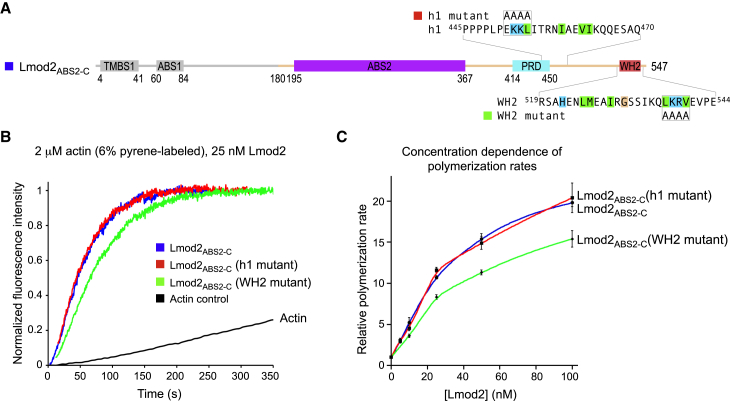
Figure 5.
Mutating the WH2 domain but not helix h1 affects the nucleation activity. (A) Domain diagram of Lmod2, showing the sequences and mutations of helix h1 and the WH2 domain. The canonical residues of the WH2 domain implicated in interactions with actin and their proposed equivalents in the inverted helix h1 are background-highlighted according to their chemical character. The two mutants tested here target the conserved LKKV motif of the WH2 domain (537LKRV540) and the corresponding sequence in helix h1 (451EKKL454), which were both mutated to AAAA within construct Lmod2ABS2-C (residues N180–R547). The region N-terminal to ABS2 (light gray) was not included in the constructs tested here. (B) Shown here is nucleation activity of Lmod2ABS2-C compared to those of the helix h1 and WH2 domain mutants. The figure shows the time course of polymerization of 2 μM Mg-ATP-actin (6% pyrene-labeled) in the presence of 25 nM Lmod2 constructs depicted in (A) or the actin control (as indicated). Note that in these experiments, actin alone (control) polymerized at a rate of 2 nM s−1. (C) Shown here is the dependence of the polymerization rates on the concentration of Lmod2 constructs, displayed as the mean of three independent experiments ± SE. To see this figure in color, go online.