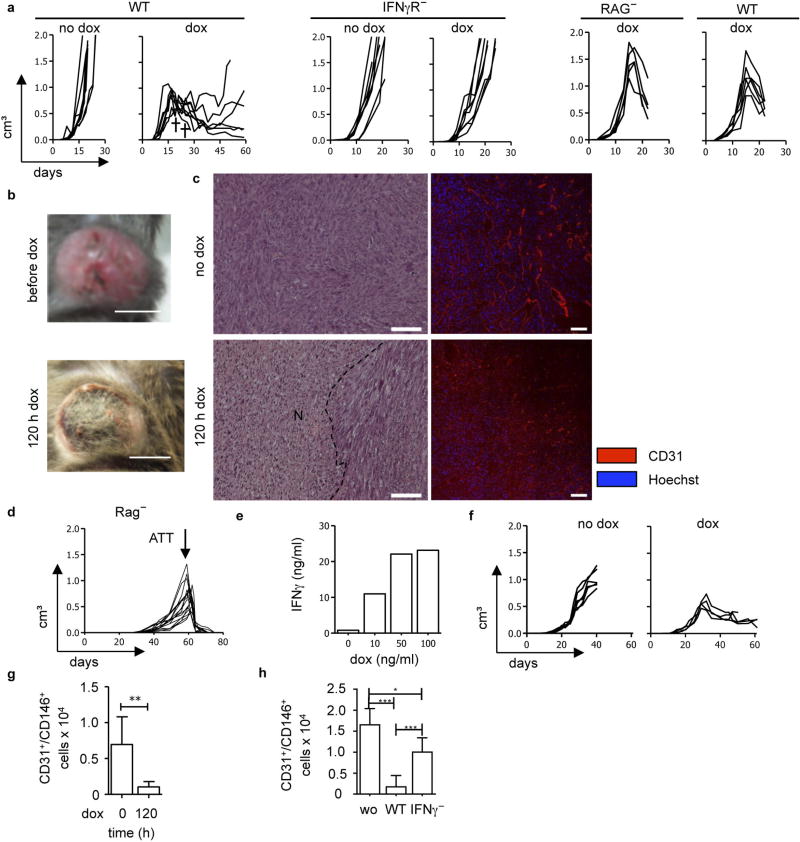
Extended Data Figure 1. Release of IFNγ in established tumours leads to necrosis, blood vessel reduction and tumour regression.
a-h, MCA313IFNγ-IND tumours (a–c), 16.113 tumours (d, h) and 16.113-999IFNγ-IND tumours (e–g). a , Without IFNγ induction, MCA313IFNγ-IND tumours grow progressively (1st panel). Dox-induced IFNγ expression in established MCA313IFNγ-IND tumours (2nd panel) leads to tumour regression in wild-type but not IFNγR− mice (3rd, 4th panel). Differences in tumour growth between ‘no dox’ and ‘dox’ groups in wild-type mice are statistically significant on days 19 (*), 23 (**) and 25 (***). IFNγ induction in wild-type mice at 642 ± 236 mm3 (the two crosses indicate mice that reached a humane endpoint and were taken out of the experiment) and in IFNγR− mice at 608 ± 130 mm3. No difference in tumour growth and IFNγ-induced tumour regression of MCA313IFNγIND tumours between Rag− and Rag-competent hosts (5th panel Rag−, n = 5; 6th panel wild type, n = 6). Dox administration at 1,304 ± 290 mm3 on day 15. b, c, MCA313IFNγ-IND tumours without and 120 h after dox. b, c, Macroscopic image (b; scale bars, 0.5 cm) and H&E staining (c) (1st row), N and dotted line indicate necrotic area (2nd row), immunohistology using anti-CD31 monoclonal antibody (scale bars, 100 µm). For H&E staining, three animals per group with 4 to 5 areas were compared and differences are statistically significant (***). d, T-cell-mediated rejection of 16.113 tumours. Mice (same as depicted in Fig. 1c) were subcutaneously injected with 106 16.113 cells. On day 57, 107 TCR transgenic T cells specific for SV40 large T antigen, epitope I (TCR-I), expressed by 16.113 tumour cells, were transferred when tumour size was 489 ± 253 mm3. Combined data from two experiments is shown (n = 16). e–g, Induction of IFNγ in established carcinomas leads to tumour regression and blood vessel reduction. e, Dox-dependent IFNγ expression by 16.113-999IFNγ-IND cells in vitro, analysed by ELISA (mean values from two experiments are shown). f, g, Dox-induced IFNγ expression in established 16.113-999IFNγ-IND tumours grown in Rag− mice leads to tumour regression (f) and blood vessel reduction (g). The relative number of tumour endothelial cells (CD31+CD146+) without and 120 h after IFNγ induction in tumours was determined from 107 tumour cells by flow cytometry. h, IFNγ released during T-cell-mediated rejection of 16.113 tumours contributes to blood vessel reduction. Tumours were established as in d, and either not treated, treated with 106 TCR-I-transduced T cells from either wild-type or IFNγ− mice. On day 5 after ATT, the relative number of tumour endothelial cells (CD31+CD146+) was determined as in g *P < 0.05, **P < 0.01 and ***P < 0.001. The number of mice, replications and sample size for each experiment are shown in Supplementary Table 3.